Abstract
Background
Curcumin is a component of Curcuma longa with various biological activities. The present study aimed to investigate curcumin’s inhibitory effects on epithelial-mesenchymal transition (EMT) in colorectal cancer (CRC) cells and possible mechanisms of action underlying these effects.
Material/Methods
Human SW480 CRC cells were incubated with curcumin at 0.1, 0.2, 0.4, 0.8, or 1.6 μmol/L. The 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay was utilized to evaluate cell viabilities. The DNA methylation levels of the cdx2 promoter were assessed by bisulfite sequencing polymerase chain reaction (BSP). Real-time quantitative PCR was used to measure the mRNA expression levels. Protein expression levels were evaluated with western blotting. Immunofluorescence staining was used to evaluate the nuclear translocation of β-catenin.
Results
Curcumin concentrations of 0.1, 0.2, and 0.4 μmol/L showed no significant association with the viability of SW480 cells, which were chosen for subsequent experiments. Curcumin incubation significantly downregulated expression levels of DNA methyltransferase1 (DNMT1), DNMT3a, and the methylation levels of the cdx2 promoter in a concentration-dependent manner. The expression levels of N-cadherin, Vimentin, Wnt3a, Snail1, and Twist, as well as the nuclear translocation levels of β-catenin, were reduced in a curcumin concentration-dependent manner. The expression levels of E-cadherin were increased in a curcumin concentration-dependent manner.
Conclusions
Curcumin negatively regulated transcription factors promoting EMT in CRC cells by decreasing cdx2 promoter DNA methylation and consequently suppressing the CDX2/Wnt3a/β-catenin signaling pathway.
MeSH Keywords: Colorectal Neoplasms, Curcumin, Epithelial-Mesenchymal Transition, Wnt Signaling Pathway
Background
According to the worldwide report Global Cancer Statistics, the incidence of colorectal cancer (CRC) has been continuously increasing and is now the second most common malignant tumor in East Asia [1]. The prognosis for CRC is relatively poor due to limited treatments for high-grade CRC, which has a 5-year survival rate of below 10% [2]. Chemotherapy is currently offered as the treatment of “reluctant choice” for high-grade CRC because high recurrence and serious side effects limit the clinical application of chemotherapeutic agents such as oxaliplatin and capecitabine [3]. Thus, a better understanding of the molecular mechanisms underlying CRC and an exploration of more effective agents are of great clinical significance.
The malignancy of CRC is related to many cellular pathological activities, such as proliferation, migration, morphogenesis, and epithelial-mesenchymal transition (EMT) [4]. EMT is a process which facilitates the acquisition of mesenchymal properties by cells and the loss of their epithelial characteristics. The role of EMT in the promotion of invasion and metastasis of human malignant cancer cells has been accepted [5]. The regulation of EMT is very complicated and various signaling pathways are thought to be involved.
Evidenced by previous investigations, β-catenin acts as a key regulator of the EMT process by playing multiple roles [6]. For instance, β-catenin is reported as a transcription factor activating expression of targeted genes, such as c-Myc and MMP7, that participate in invasion and metastasis [7]. Moreover, β-catenin acts as a molecular chaperone, facilitating the association between cytoskeleton and cadherins to improve cell adhesion. The Wnt pathway is the upstream signaling pathway regulating β-catenin [8]. Results from previous studies have indicated that upregulation of Wnt3a promotes the nuclear translocation of β-catenin [9]. Several recent studies have suggested significant correlations between CRC malignancy and caudal type homeobox 2 (CDX2), which is thought to act as a tumor suppressor [10]. CDX2 has also been implicated as a mediator of Wnt signaling [11]. Thus, CDX2/Wnt/β-catenin signaling could be a potential molecular target for the regulation of EMT.
Curcumin (C21H20O6) is one of the active components of the turmeric root, Curcuma longa, which is used in Traditional Chinese Medicine. The anti-cancer activity of curcumin against human CRC has been supported by many investigations [12]. Curcumin regulates protein expression via effects on DNA methyltransferases (DNMTs) [13]. Notably, cdx2 promoter methylation is altered in human malignant cancer cells [14]. Therefore, it is reasonable for us to speculate that curcumin may decrease the methylation status of cdx2 via DNMTs, resulting in deactivation of the Wnt/β-catenin pathway and thereby leading to suppression of EMT in CRC cells. To test our hypothesis, in the present study, human CRC cells were exposed to curcumin at below-cytotoxic concentrations. DNA methylation of the cdx2 promoter, activation of CDX2/Wnt/β-catenin signaling, and expression levels of EMT markers were investigated. The results of our study provide new clues concerning novel therapeutic molecular targets for CRC and new evidence supporting the application of curcumin in the treatment of CRC.
Material and Methods
Cells and treatments
Human CRC cell line SW480 was provided by the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and maintained in PRMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with fetal bovine serum (10%, FBS, Gibco, Grand Island, NY, USA) and mixed antibiotics (100 U/ml penicillin and 0.1 mg/ml streptomycin; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Cells were maintained in a cell incubator (Thermo, Waltham, MA, USA) that provided a humidified atmosphere of 95% fresh air and 5% CO2 at 37°C. Curcumin (Sigma-Aldrich, St. Louis, MO, USA) at 0, 1, 2, or 4 μmol/L was used to treat the cells for 48 h.
Cell viability assessment
Cell viability was assessed by 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay according to the protocol described in previous studies [15]. SW480 cells, at a density of 1×104 cells per well, were treated with curcumin at 0, 0.1, 0.2, 0.4, 0.8, or 1.6 μmol/L for 24 h. An MTT assay kit (Beyotime, Shanghai, China) was used to carry out the assay. The optical density at 490 nm was read with a microplate reader (Bio-Rad, Hercules, CA, USA). The cell viability was calculated by using the formula (ODtreatment/ODcontrol)×100%.
Methylation evaluation for the cdx2 promoter
The methylation level of the cdx2 promoter was evaluated by bisulfite sequencing polymerase chain reaction (BSP) according to the descriptions in previous studies [16]. Genomic DNA was extracted from cells using a proteinase-K method with a DNA extraction kit (TianGen, Beijing, China). The content and purity of DNA was verified with an ultraviolet spectrophotometer. Extracted DNA was precipitated in ethanol, dissolved in low-TE buffer (10 mmol/L EDTA, 100 mmol/L Tris-HCl, pH 8.0) and stored at −20°C. Extracted genomic DNA was bisulfite modified using an EpiTect Bisulfite kit (Qiagen, Duesseldorf, Germany) per the manufacturer’s instructions. Methylation-specific polymerase chain reaction primers for cdx2 were designed and synthesized by GenePharma (Shanghai, China). The sequences were: forward 5′-TTTTCGTGTTTTTCGGTAGTTTTTAGC-3′, reverse 5′-ACTCACGTACATAATAACGAAAATCCG-3′. The reaction conditions were: pre-denaturation for 15 min at 95°C, followed by 50 cycles of denaturation for 30 s at 95°C, annealing for 30 s at 57°C, and extension for 20 s at 72°C. The PCR products were purified using a QIAquick purification kit (Qiagen, Duesseldorf, Germany). After sequencing, the methylation of the cdx2 promoter was viewed and analyzed using CpG Viewer software (Insilicase Boinformatics, USA).
Immunofluorescent staining
The nuclear translocation of β-catenin was evaluated by immunofluorescent staining. SW480 cells were grown on cover glass and were then fixed with 10% formaldehyde and incubated with blocking buffer (Abcam, Cambridge, MA, USA) for 30 min. Cells were then incubated with primary antibody against β-catenin (1: 100, Abcam, Cambridge, MA, USA) at 4°C for 8 h. After PBS washing, the cells were further incubated with Alexa488-conjugated secondary antibody (1: 200, Abcam, Cambridge, MA, USA) for 20 min. The nuclei were stained with DAPI (Beyotime, Shanghai, China). An inverted fluorescence microscope was used to observe the fluorescent images.
Real-time quantitative PCR
Total RNA was extracted from the cultured SW480 cells using a TRIzol Reagent Extraction kit (Invitrogen, Carlsbad, CA, USA) according to the protocol provided by the manufacturer. Primers for cdx2, wnt3a, snail, twist, e-cadherin, n-cadherin, vimentin, and gapdh are listed in Table 1. A BeyoRT™ cDNA kit (Beyotime, Shanghai, China) was used to conduct the reverse transcription of cDNA. Amplifications were performed using the 7300 Real-Time PCR system (Applied Biosystems, Waltham, MA, USA) and SYBR Green PCR kit (Invitrogen, Carlsbad, CA, USA). The reaction conditions were: pre-denaturation for 4 min at 95°C followed by 30 cycles of denaturation for 30 s at 95°C, annealing for 30 s at 57°C, and extension for 30 s at 72°C. Fold-changes of transcription levels for the targeted genes were determined using the 2−ΔΔCt method, with gapdh as the internal reference.
Table 1.
Primer sequences for RT-PCR.
| Gene names | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| cdx2 | CCACGCTGGGGCTCTCT | GTCTAGCAGAGTCCACGCTC |
| wnt3a | CCATCCTCTGCCTCAAATTC | TGGACAGTGGATATAGCAGCA |
| snail1 | TCGGAAGCCTAATACAGCGA | AGATGAGCATTGGCAGCGAG |
| twist | CGACGACAGCCTGAGCAACA | CCACAGCCCGCAGACTTGTT |
| e-cadherin | CTGAGAACGAGGCTAACG | GTCCACCATCATCATT |
| n-cadherin | TGAAGTCCCCAATGT CTC CA | GCATCATCATCCTGCTTATCC |
| vimentin | GAGAACTTTGCCGTTGAAGC | GCTTCCTGTAGGTGGCAATC |
| gapdh | GGTGAAGGTCGGAGTCAACG | CAAAGTTGTCATGGATGAACC |
Western blotting
Cells were harvested and lysed using the cell lysis buffer system (Beyotime, Shanghai, China). Total and nuclear protein were extracted by using the Total Protein Extraction kit (Beyotime, Shanghai, China) and Nuclear Protein Extraction kit (Beyotime, Shanghai, China), respectively. After centrifugation at 12 800 g at 4°C for 20 min, the extracted protein samples were subjected to concentration determination with a bicinchoninic acid (BCA) protein assay kit (Beyotime, Shanghai, China). Then, the proteins were vertically separated by 12% SDS-PAGE and electronically transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After blocking with protein blocking buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h, the protein samples were incubated with primary antibodies against CDX2 (1: 2000, Abcam, Cambridge, MA, USA), Wnt3a (1: 2000, CST, Danvers, MA,USA), β-catenin (1: 2000, Abcam, Cambridge, MA, USA), Snail1 (1: 1000, Abcam, Cambridge, MA, USA), Twist (1: 1000, Abcam, Cambridge, MA, USA), E-cadherin (1: 2000, Abcam, Cambridge, MA, USA), N-cadherin (1: 2000, Abcam, Cambridge, MA, USA), Vimentin (1: 2000, Abcam, Cambridge, MA, USA), and GAPDH (1: 4000, Abcam, Cambridge, MA, USA) for 8 h at 4°C. After washing, the membranes were further incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 2 h at room temperature. Then, the immunoblots were visualized and analyzed with an enhanced chemiluminescence detection system (GE Healthcare, Little Chalfont, UK) per the manufacturer’s instructions.
Statistics
All experiments were repeated 6 times (n=6). Data acquired were presented as mean ± standard deviation. Differences between groups were analyzed with one-way analysis of variance (ANOVA). The Student Newman-Keuls (NSK) test was applied as a post-hoc test. Differences were considered statistically significant at P<0.05.
Results
Determination of the Concentrations at which Curcumin does not Inhibit SW480 Viability
To eliminate effects on viability due to inhibition of EMT, non-inhibitory concentrations of curcumin on SW480 cells were determined by MTT assay. As demonstrated in Figure 1, curcumin began to show significant inhibitory effects on viability of SW480 cells starting at 0.8 μmol/L. Thus, curcumin concentrations of 0.2, 0.4, and 0.8 μmol/L were selected for subsequent experiments.
Figure 1.
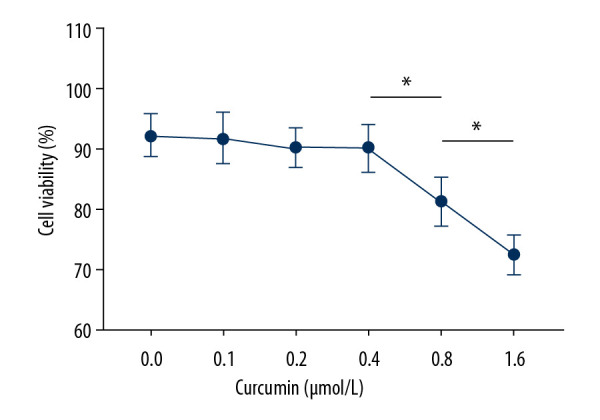
Line chart indicating relative cell viabilities of SW480 cells incubated with curcumin at 0, 0.1, 0.2, 0.4, 0.8, or 1.6 μmol/L for 24 h. (n=6, * P<0.05).
Curcumin incubation inhibited EMT of SW480 cells in a concentration-dependent manner
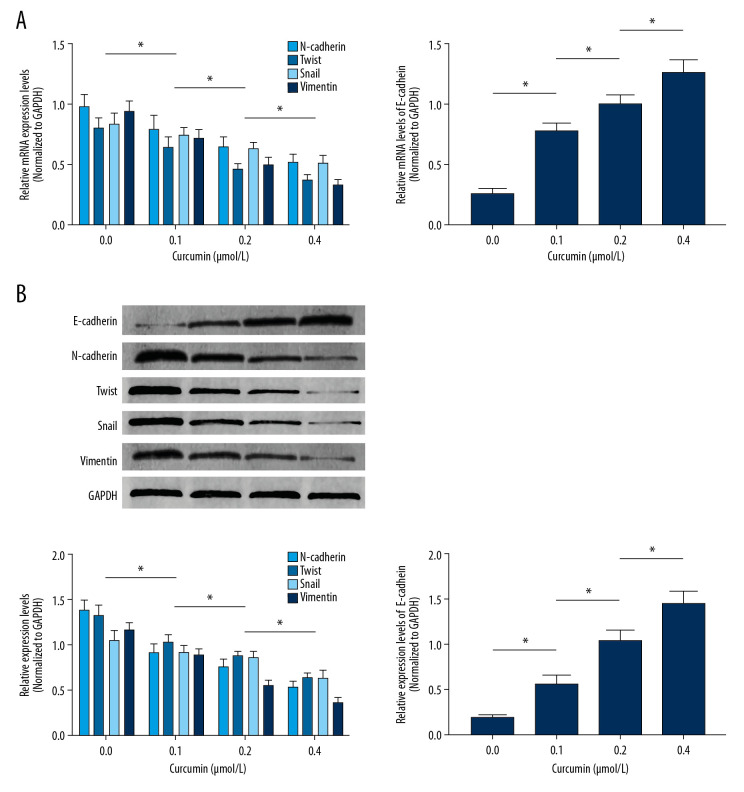
The EMT-related proteins E-cadherin and N-cadherin, as well as β-catenin-targeted gene expression levels, were used to evaluate the inhibitory effects of curcumin on SW480 EMT. As demonstrated in Figure 2A, curcumin treatment significantly upregulated mRNA expression levels of e-cadherin and downregulated mRNA expression levels of n-cadherin, snail, twist, and vimentin in a concentration-dependent manner. Figure 2B shows the protein expression levels, as evidenced by immunoblots. Curcumin administration dramatically increased E-cadherin expression but decreased expression levels of N-cadherin, Twist, Snail, and Vimentin in SW480 cells in a concentration-dependent manner.
Figure 2.
(A) Columns indicating the relative mRNA expression levels of n-cadherin, twist, snail, vimentin, and e-cadherin in SW480 cells incubated with curcumin at 0, 0.1, 0.2, or 0.4 μmol/L for 24 h. (B) Immunoblots of E-cadherin, N-cadherin, Twist, Snail, Vimentin, and GAPDH (upper panel). Columns (lower panel) indicate the relative expression levels of E-cadherin, N-cadherin, Twist, Snail, and Vimentin in SW480 cells incubated with curcumin at 0, 0.1, 0.2, or 0.4 μmol/L for 24 h. (n=6, * P<0.05)
Curcumin incubation reduced cdx2 promoter methylation by affecting the expression of DNMTs
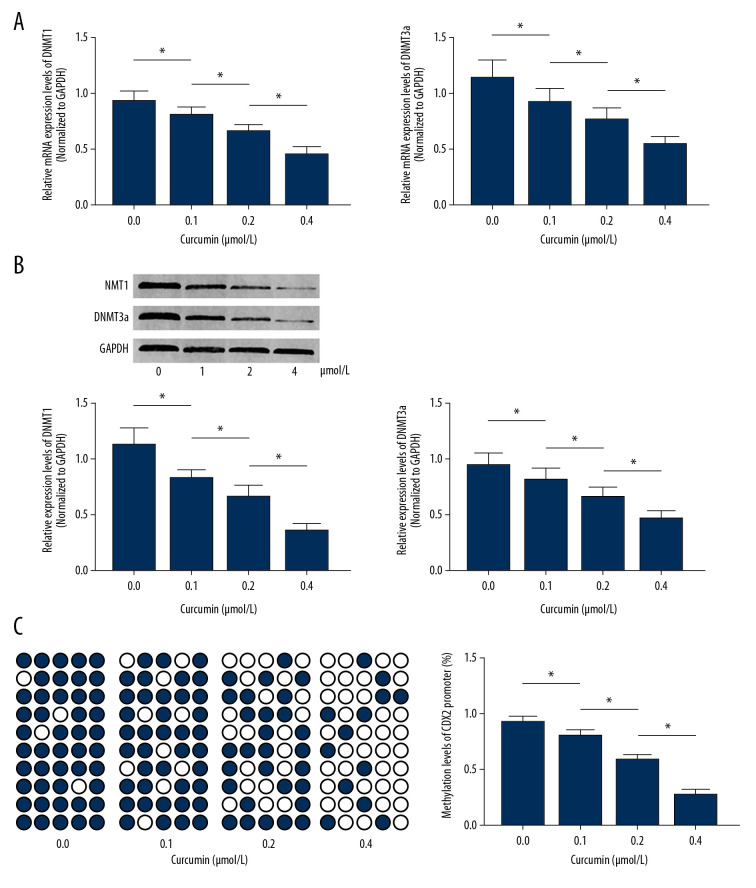
As demonstrated in Figure 3A and 3B, curcumin incubation significantly decreased both mRNA and protein expression levels of DNMT1 and DNMT3a in SW480 cells in a concentration-dependent manner. Figure 3C shows the methylation of the cdx2 promoter, as assayed using the BSP method. Results of the BSP analysis suggested that curcumin decreased the methylation levels of the cdx2 promoter in SW480 cells in a concentration-dependent manner.
Figure 3.
(A) Columns indicate the relative mRNA expression levels of dnmt1 and dnmt3a in SW480 cells incubated with curcumin at 0, 0.1, 0.2, or 0.4 μmol/L for 24 h. (B) Immunoblots of DNMT1, DNMT3a, and GAPDH. Columns indicate the relative expression levels of DNMT1 and DNMT3a in SW480 cells incubated with curcumin at 0, 0.1, 0.2, or 0.4 μmol/L for 24 h. (C) cdx2 promoter DNA methylation levels. Black dots indicate methylation sites while white dots indicate unmethylated cdx2 promoter sites. Columns indicate the DNA methylation rate of the cdx2 promoter in SW480 cells incubated with curcumin at 0, 0.1, 0.2, or 0.4 μmol/L for 24 h. (n=6, * P<0.05)
Curcumin incubation enhanced the inhibitory effects of CDX2 on the Wnt/β-catenin pathway in SW480 cells
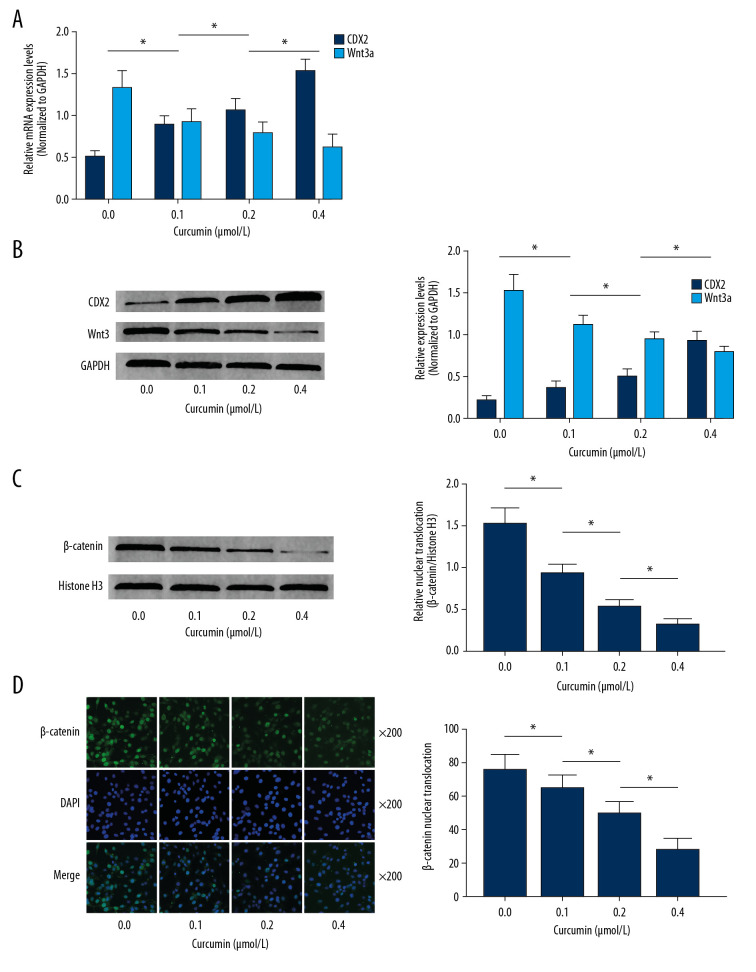
As demonstrated in Figure 4A and 4B, the expression levels of CDX2 and Wnt3a were significantly upregulated in SW480 cells incubated with curcumin, in a concentration-dependent manner. β-catenin expression levels in nuclear protein were also increased by curcumin incubation in a concentration-dependent manner (Figure 4C). As demonstrated in Figure 4D, immunofluorescence staining confirmed that nuclear translocation of β-catenin was significantly attenuated by curcumin incubation in a concentration-dependent manner.
Figure 4.
(A) Columns indicate the relative mRNA expression levels of cdx2 and wnt3a in SW480 cells incubated with curcumin at 0, 0.1, 0.2, or 0.4 μmol/L for 24 h. (B) Immunoblots of CDX2, Wnt3a, and GAPDH. Columns indicate the relative expression levels of CDX2 and Wnt3a in SW480 cells incubated with curcumin at 0, 0.1, 0.2, or 0.4 μmol/L for 24 h. (C) Immunoblots of β-catenin and histone H3. Columns indicate the relative nuclear translocation levels of β-catenin (β-catenin/histone H3) in SW480 cells incubated with curcumin at 0, 0.1, 0.2, or 0.4 μmol/L for 24 h. (D) Captured images of immunofluorescence staining of β-catenin (stained by Alexa-488, green fluorescence), cell nuclei (stained by DAPI, blue fluorescence) and their merge were demonstrated. Columns indicate the relative nuclear translocation levels (translocated cell count/total cell count) in SW480 cells incubated with curcumin at 0, 0.1, 0.2, or 0.4 μmol/L for 24 h. (n=6, * P<0.05).
Discussion
CRC is one of the most common malignant tumors of the digestive tract, and the prognosis for CRC is poor because CRC is often found at advanced, or even late, stages. The invasion and metastasis of CRC limits the application of surgical resection, which is believed to be the only effective therapy. The pathological process of invasion and metastasis by CRC can happen at early stages, and this is highly associated with EMT [17]. By converting cellular characteristics of epithelial cells into mesenchymal attributes, EMT accelerates tumor progression by strengthening invasion ability, migration, and metastasis of the cancer cells [18].
CDX2 was initially reported as a critical mediator regulating gut development and homeostasis. Recent investigations have suggested a strong association between CDX2 and CRC [19]. Lower CDX2 expression indicates poorer prognosis and predicts inferior therapeutic responses to chemotherapy in CRC [20]. In the present study, CDX2 promoter methylation level was found to be dramatically upregulated, resulting in a significant downregulation of CDX2 expression in SW480 cells. Previous studies have indicated a role for CDX2 in regulating Wnt signaling pathway activation. This pathway is thought to be hyperactivated in the development of several human cancers. It has been reported that CDX2 knockdown leads to activation of the Wnt signaling pathway, which facilitates the invasion and migration of human colon cells [21].
The molecular mechanisms underlying EMT and its regulation are very complicated. As an evolutionarily conserved pathway, Wnt signaling plays an important role in maintaining cellular homeostasis. A family member of Wnt, Wnt3a, was found to play key regulatory roles in several human malignant cancers. Wnt3a interacts with Wnt co-receptor Frizzled (Fz) through contact with low-density lipoprotein receptors, leading to nuclear translocation of β-catenin [22]. Beta-catenin facilitates initiation of its targeted genes in the nucleus by binding to Lef/Tcf transcription factors. Wnt/β-catenin signaling pathway activation is thought to be involved in EMT because some EMT-associated proteins, namely Twist and Snail1, are thought to be target genes of β-catenin [23]. In the present study, the Wnt/β-catenin signaling pathway was found to be activated in SW480 cells. As a result, the expression levels of Twist and Snail1 were upregulated, which is recognized as a hallmark of EMT.
The anti-cancer activity of curcumin is well accepted, and many explanatory molecular mechanisms have been proposed. A previous investigation showed that curcumin suppresses gastric cancer cells through inhibiting the Wnt/β-catenin signaling pathway [24]. A recent study reported that curcumin inhibits the Wnt/β-catenin pathway by increasing CDX2 expression in CRC cells [15]. In the present investigation, non-cytotoxic concentrations of curcumin were used to treat SW480 cells to avoid cell death-related gene expression changes. The expression of CDX2 was upregulated in a concentration-dependent manner. Notably, the expression levels of DNMT1 and DNMT3a were reduced after curcumin incubation, indicating that curcumin possibly upregulates CDX2 expression by affecting methylation of its promoter. Indeed, by the BSP technique, we confirmed that curcumin treatment decreased CDX2 promoter methylation. As a result, in accordance with previous studies, we conclude that curcumin treatment decreases the activation of Wnt/β-catenin signaling-mediated EMT in SW480 cells.
Conclusions
Our key finding was that curcumin inhibited EMT in CRC cells. CDX2 promoter methylation levels were reduced by curcumin and this increased CDX2 expression levels. As a result, activation of Wnt/β-catenin signaling-mediated EMT was suppressed. CDX2 promoter methylation may serve as a novel therapeutic target for the treatment of CRC. Moreover, our results provided new evidence supporting the clinical application of curcumin or curcumin-containing drugs as treatments for CRC patients.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Zhejiang Provincial TCM Science and Technology Program of China (2020ZQ015)
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 3.Fakih MG. Metastatic colorectal cancer: Current state and future directions. J Clin Oncol. 2015;33:1809–24. doi: 10.1200/JCO.2014.59.7633. [DOI] [PubMed] [Google Scholar]
- 4.Cao H, Xu E, Liu H, et al. Epithelial-mesenchymal transition in colorectal cancer metastasis: A system review. Pathol Res Pract. 2015;211:557–69. doi: 10.1016/j.prp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Xie R, Wang J, Tang W, et al. Rufy3 promotes metastasis through epithelial-mesenchymal transition in colorectal cancer. Cancer Lett. 2017;390:30–38. doi: 10.1016/j.canlet.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/β-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur J Cancer. 2015;51:1638–49. doi: 10.1016/j.ejca.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Mishra J, Das JK, Kumar N. Janus kinase 3 regulates adherens junctions and epithelial mesenchymal transition through β-catenin. J Biol Chem. 2017;292:16406–19. doi: 10.1074/jbc.M117.811802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Schinzari V, Timperi E, Pecora G, et al. Wnt3a/β-catenin signaling conditions differentiation of partially exhausted T-effector cells in human cancers. Cancer Immunol Res. 2018;6:941–52. doi: 10.1158/2326-6066.CIR-17-0712. [DOI] [PubMed] [Google Scholar]
- 10.Bruun J, Sveen A, Barros R, et al. Prognostic, predictive, and pharmacogenomic assessments of CDX2 refine stratification of colorectal cancer. Mol Oncol. 2018;12:1639–55. doi: 10.1002/1878-0261.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan H-B, Zhai Z-Y, Li X-G, et al. CDX2 stimulates the proliferation of porcine intestinal epithelial cells by activating the mTORC1 and Wnt/β-catenin signaling pathways. Int J Mol Sci. 2017;18:2447. doi: 10.3390/ijms18112447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deguchi A. Curcumin targets in inflammation and cancer. Endocr Metab Immune Disord Drug Targets. 2015;15:88–96. doi: 10.2174/1871530315666150316120458. [DOI] [PubMed] [Google Scholar]
- 13.Maugeri A, Mazzone MG, Giuliano F, et al. Curcumin modulates DNA methyltransferase functions in a cellular model of diabetic retinopathy. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/5407482. 5407482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu Y, Dang S, Hou P. Gene methylation in gastric cancer. Clin Chim Acta. 2013;424:53–65. doi: 10.1016/j.cca.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Jiang X, Li S, Qiu X, et al. Curcumin inhibits cell viability and increases apoptosis of SW620 human colon adenocarcinoma cells via the caudal type homeobox-2 (CDX2)/Wnt/β-catenin pathway. Med Sci Monit. 2019;25:7451–58. doi: 10.12659/MSM.918364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H-B, Wei H, Wang J-S, et al. Down-regulated expression of LINC00518 prevents epithelial cell growth and metastasis in breast cancer through the inhibition of CDX2 methylation and the Wnt signaling pathway. Biochim Biophys Acta Mol Basis Dis. 2019;1865:708–23. doi: 10.1016/j.bbadis.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Vu T, Datta PK. Regulation of EMT in colorectal cancer: A culprit in metastasis. Cancers. 2017;9:171. doi: 10.3390/cancers9120171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suarez-Carmona M, Lesage J, Cataldo D, Gilles C. EMT and inflammation: Inseparable actors of cancer progression. Mol Oncol. 2017;11:805–23. doi: 10.1002/1878-0261.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbareschi M, Murer B, Colby TV, et al. CDX-2 homeobox gene expression is a reliable marker of colorectal adenocarcinoma metastases to the lungs. Am J Surg Pathol. 2003;27:141–49. doi: 10.1097/00000478-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Zhang BY, Jones JC, Briggler AM, et al. Lack of caudal-type homeobox transcription factor 2 expression as a prognostic biomarker in metastatic colorectal cancer. Clin Colorectal Cancer. 2017;16:124–28. doi: 10.1016/j.clcc.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Coskun M, Olsen AK, Bzorek M, et al. Involvement of CDX2 in the cross talk between TNF-α and Wnt signaling pathway in the colon cancer cell line Caco-2. Carcinogenesis. 2014;35:1185–92. doi: 10.1093/carcin/bgu037. [DOI] [PubMed] [Google Scholar]
- 22.He Q, Yan H, Wo D, et al. Wnt3a suppresses Wnt/β-catenin signaling and cancer cell proliferation following serum deprivation. Exp Cell Res. 2016;341:32–41. doi: 10.1016/j.yexcr.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Mahmood MQ, Walters EH, Shukla SD, et al. β-catenin, twist and snail: Transcriptional regulation of EMT in smokers and COPD, and relation to airflow obstruction. Sci Rep. 2017;7:10832–40. doi: 10.1038/s41598-017-11375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng R, Deng Q, Liu Y, Zhao P. Curcumin inhibits gastric carcinoma cell growth and induces apoptosis by suppressing the Wnt/β-catenin signaling pathway. Med Sci Monit. 2017;23:163–71. doi: 10.12659/MSM.902711. [DOI] [PMC free article] [PubMed] [Google Scholar]