Figure 2.
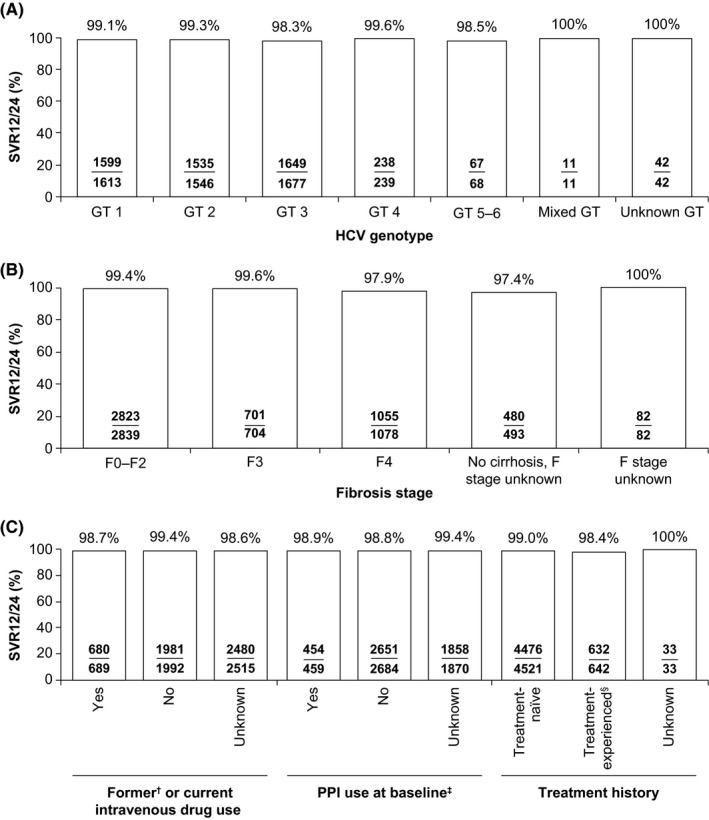
SVR12/24 in the effectiveness population analysis. Percentage of patients achieving SVR12/24 in the effectiveness population after being treated with SOF/VEL for 12 weeks without ribavirin, stratified by (A) HCV genotype (B) fibrosis stage (C) intravenous drug use, PPI use at baseline and treatment history. †The definition of former drug use varied between cohorts with respect to timing and this level of detail was not available for most patients. ‡Information on patients achieving SVR12/24 by PPI use at baseline was not available in one cohort, and thus patients from this cohort (n = 183) were not considered in this subgroup analysis. §Patients were treated with PEG‐IFN + RBV (± PI boceprevir, telaprevir, simeprevir). F, fibrosis; GT, genotype; PEG‐IFN, pegylated interferon; PI, protease inhibitor; PPI, proton pump inhibitor; RBV, ribavirin; SOF/VEL, sofosbuvir/velpatasvir; SVR12/24, sustained virological response 12/24 weeks after the end of treatment
