Figure 3.
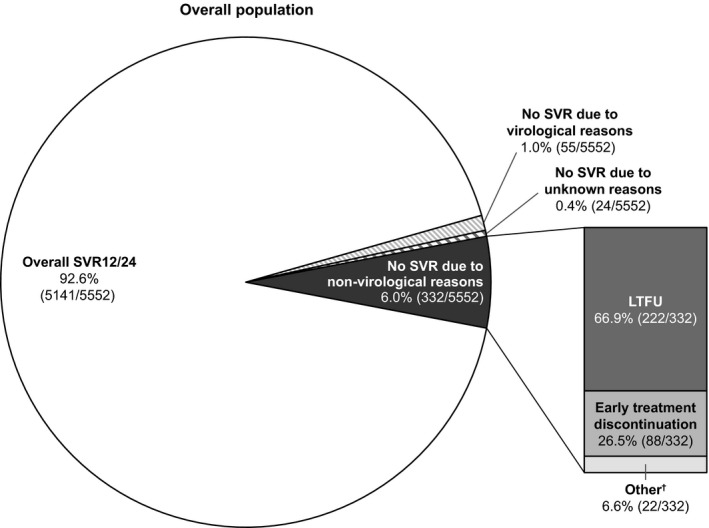
SVR12/24 outcomes in the overall patient population. Treatment outcomes in the overall patient population treated with SOF/VEL for 12 weeks without RBV. †Other non‐virological reasons were: death (5.1%; 17/332), consent withdrawal (0.3%; 1/552), non‐adherence (0.6%; 2/332) and reinfection (0.6%; 2/332). LFTU, lost to follow‐up; RBV, ribavirin; SOF/VEL, sofosbuvir/velpatasvir; SVR12/24, sustained virological response 12/24 weeks after the end of t`reatment
