Figure 3.
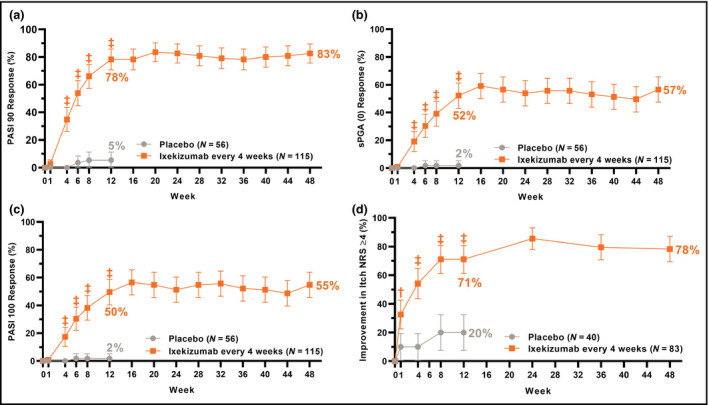
Proportions of patients achieving (a) ≥ 90% improvement in Psoriasis Area and Severity Index (PASI 90); (b) static Physician's Global Assessment score of 0 [sPGA (0)]; (c) PASI 100 and (d) ≥ 4‐point improvement from baseline in itch numerical rating scale (Itch NRS) for up to 48 weeks of treatment with ixekizumab (nonresponder imputation). Ixekizumab vs. placebo P‐values during the 12‐week double‐blind treatment period are indicated as †P < 0·01, ‡P < 0·001. Error bars indicate 95% confidence intervals. Analyses of PASI 90, sPGA (0), PASI 100 and Itch NRS ≥ 4 at week 12 (gated secondary endpoints) were included in the gated multiple testing strategy. Analyses at other timepoints were not adjusted for multiplicity.
