Figure 1.
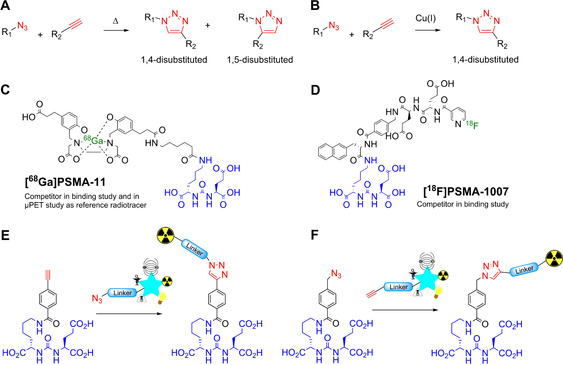
Overview of the [3+2] cycloadditions, clinically used prostate cancer radiotracers and the molecular platforms presented in this study. (A) Thermal azide–alkyne Huisgen [3+2] cycloaddition.4 (B) The copper(I)‐catalyzed azide–alkyne cycloaddition (CuAAC).4 (C) Structure of [68Ga]PSMA‐11 with the chelator HBED‐CC and the glutamate‐urea‐lysine (Glu‐urea‐Lys) motif (highlighted in blue) that binds to the prostate‐specific membrane antigen (PSMA).50 (D) Structure of [18F]PSMA‐1007.48 (E) Principle of a modular imaging agent consisting an alkyne‐functionalized Glu‐urea‐Lys motif that can be ‘clicked’ to a selected signaling moiety with azide‐functionality. The signaling moiety is chosen out of the range of different moieties, represented as the star, that is required for the aimed medical imaging application. The here presented study is showcasing its application in PET imaging. (F) The same principle of modular imaging agents using an azide‐functionalized Glu‐urea‐Lys motif52 to cover various suitable functionalized medical imaging moieties.
