Figure 6.
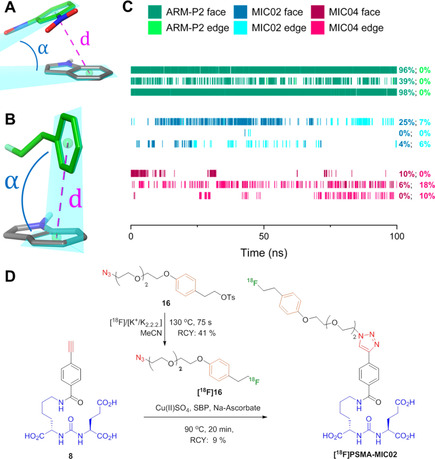
Analysis of the π−π stacking of Trp541 and the additional aromatic ring in F‐PSMA‐MIC02 and F‐PSMA‐MIC04 and the radiolabeling of the strongest binder in this study. (A) Example of a face‐to‐face π−π stacking between dinitrophenyl (DNP, green) and Trp541 (gray) from the complex of ARM‐P2 with PSMA (PDB ID: 2XEI). (B) Example of an edge‐to‐face π−π interaction between the additional electron‐rich ring (green) and Trp541 (gray) from the second MD run of F‐PSMA‐MIC04 (frame number 282). The ring distance and ring angle measurements are illustrated as pink dotted lines and blue arcs, respectively. In all the structures, carbon atoms are colored as indicated above, and other atoms are colored blue (nitrogen), red (oxygen) and light green (fluorine). (C) Timeline representation of the π−π interactions in the three MD runs of ARM‐P2 (green), F‐PSMA‐MIC02 (blue) and F‐PSMA‐MIC04 (red). Dark colors indicate face‐to‐face interactions and bright colors indicate edge‐to‐face interactions. On the right side, the frequency of the interactions for individual runs is reported with the same coloring. (D) The automated synthesis route of [18F]PSMA‐MIC02 using the FlowSafe radiosynthesis module.
