Abstract
Objective
The objective of this study was to investigate the safety and feasibility of treating infrapopliteal lesions using a novel drug delivery catheter locally delivering liquid paclitaxel.
Background
Balloon angioplasty is currently the Gold Standard to treat below‐the‐knee disease; however, restenosis continues to be a great challenge following these percutaneous revascularization procedures.
Methods
The Occlusion Perfusion Catheter for Optimal Delivery of Paclitaxel for the Prevention of Endovascular Restenosis (COPPER‐A) study—Below‐the‐Knee Cohort was a prospective, nonrandomized, multicenter, feasibility, and safety study that enrolled 35 patients at 11 participating sites. The safety endpoints at 1, 3, and 6 months were freedom from thrombosis, major amputation in the target limb and target limb related death. The efficacy endpoints were primary patency and freedom from clinically driven target lesion revascularization at 6 months.
Results
All patients tolerated the procedure well with no reports of adverse procedural events. Thirty‐five patients were treated with a mean lesion length of 112 ± 81.2 mm with the lesion length range of 20–286 mm. At 6‐month follow‐up, primary patency was 89.3% and freedom from clinically driven target lesion revascularization was 96.4%. No patients demonstrated thrombosis, major amputation in the target limb and target limb related death at the 1‐, 3‐ and 6‐months follow‐up intervals.
Conclusions
The results of this multi‐center study demonstrated that infrapopliteal arteries can be safely and effectively treated with liquid paclitaxel using the occlusion perfusion catheter.
Keywords: clinical trial, paclitaxel, peripheral intervention
1. INTRODUCTION
Percutaneous transluminal angioplasty is the most common method of endovascular treatment for infrapopliteal atherosclerotic disease and its most severe manifestation is critical limb ischemia (CLI).1, 2, 3, 4 CLI is a leading cause of major amputation and a significant cause of morbidity and mortality.5 Treatment of these below‐the‐knee (BTK) vessels are among the greatest challenge for the endovascular interventionalist due to the high incidence of long chronic total occlusions and calcified lesions with poor distal runoff. Historically, the treatment of these vessels represents a considerable challenge for interventionalist with high restenosis rates and poor long‐term clinical patency.6
The recent advent of drug coated balloons (DCBs) was to improve patency for BTK interventions.7, 8 DCBs deliver paclitaxel directly to the luminal surface of treated vessels to inhibit neointimal formation and reduce restenosis. Clinical trials of DCBs have shown promising results in the treatment of femoropopliteal disease; however, in the BTK arteries, results have been limited and long‐term success has yet to be determined. The IN.PACT DEEP trial demonstrated unsatisfactory results to treat patients with CLI with comparable efficacy rates of the DCB to balloon angioplasty.9 The BIOLUX P‐II trial also showed similar patency loss between DCB and balloon angioplasty at 12 months in patients with CLI.10 In a more recent BTK single‐center clinical study, DCB demonstrated overall target lesion revascularization of 15.9% at a median follow‐up at 9 months.11 Overall, DCBs have not demonstrated clinical or angiographic advantage at 1 year follow‐up compared to balloon angioplasty.12
The first use of a novel perfusion catheter capable of delivering liquid paclitaxel was recently described in a first‐in‐human study.13 The Occlusion Perfusion Catheter, (PRESSANA OPC, Advanced Catheter Therapies, Chattanooga, TN) is a universal drug‐delivery catheter that can deliver liquid paclitaxel to the medial layer, treat multiple lesions with a single device and minimize any drug loss during the process. The occlusion perfusion catheter (OPC) delivers paclitaxel by creating a treatment chamber between two occlusion balloons through which the agent is delivered. The delivery of liquid paclitaxel is mechanically driven using pressure, measured in real‐time. Local liquid delivery provides a novel approach to deliver paclitaxel uniformly into the vessel wall and potentially overcomes the shortcomings of current procedures to treat BTK arterial stenosis. The aim of this study was thus to assess the feasibility, safety, and efficacy of liquid paclitaxel administrated using the OPC for the prevention of restenosis in infrapopliteal de novo and restenotic lesions.
2. MATERIALS AND METHODS
2.1. Study design
The Occlusion Perfusion Catheter for Optimal Delivery of Paclitaxel for the Prevention of Endovascular Restenosis (COPPER‐A study)—Below‐the‐Knee Cohort was a prospective, non‐randomized multicenter trial, at 11 sites, designed to assess the safety and efficacy of paclitaxel administration using the OPC for the prevention of restenosis in infrapopliteal de novo and restenotic lesions. The protocol was approved by a national institutional review board and individual site institutional review boards. All patients provided written informed consent prior to enrollment. The study was conducted in accordance with good clinical practice and applicable regulations for non‐significant risk studies.
2.2. Study population
Patients eligible for enrollment had a Rutherford classes 2–5, infrapopliteal lesions ≥20 mm in length, age ≥18 years and able to tolerate dual anti‐platelet therapy for a minimum of 1 month. General angiographic inclusion criteria included reference vessel diameter ≥2 mm and ≤4 mm, single or multiple lesions in the infrapopliteal arteries, lesion location in the region between the trifurcation of vessels to the ankle, minimum of one vessel run‐off, pre‐intervention diameter stenosis ≥70% and successful pre‐treatment therapy to achieve residual stenosis to ≤30%. Major exclusion criteria included previous intervention of the target vessel with a stent, DCB or other drug delivery catheter, pregnancy or lactating, known allergies to study medication and materials, planned amputation prior to procedure, and acute limb ischemia. Angiographic exclusion criteria included flow limiting dissection requiring stent placement, significant inflow lesion or occlusion left untreated in the ipsilateral iliac, superficial femoral artery, or popliteal artery proximal to the target lesion and visible thrombus within or proximal to the target artery.
2.3. Study device: The OPC
The FDA 510(k) cleared OPC is a 5 Fr device (0.014″ guidewire compatible) with an outer diameter of 1.67 mm. The catheter has three balloons; two compliant occlusion balloons (one proximal and one distal) which define the treatment chamber, and a center space‐occupying balloon (Figure 1). The compliant occlusion balloons are sized to minimize trauma to the vessel wall during treatment. Treatment chamber pressure is measured real‐time via a sensor located within the treatment chamber which is connected to an external pressure monitor for the purpose of monitoring the pressure inside the treatment chamber during infusion of the therapeutic agent. Therapeutic agents are infused through the treatment inflow under continuous pressure injection. Radiopaque markers are located on both sides of the occlusion balloons to define the treatment chamber to assist in catheter placement under fluoroscopy. The size of the OPC for this study was 3 mm in the diameter of the occlusion balloons (which can be expanded to 4 mm if needed) and 150 mm in treatment chamber length (3.0 mm × 150 mm) with a nominal working length of 135 cm.
Figure 1.
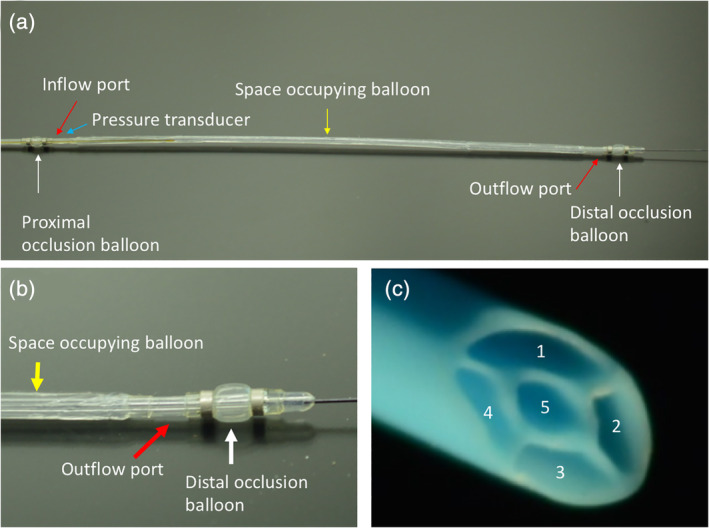
The occlusion perfusion catheter (OPC) universal drug delivery catheter. (a) The OPC, a multi‐lumen balloon catheter, is designed to temporarily occlude the target lesion from blood flow, flush the blood from the treatment chamber, and then locally deliver the therapeutic agents into the artery. A built‐in fiber optic pressure sensor continuously monitors the treatment chamber during delivery. (b) The distal end of the OPC catheter demonstrating the space occupying balloon, outflow port and distal occlusion balloon. (c) A cross‐section of the catheter showing the 5 lm that correspond to 1—proximal and distal balloons, 2—space occupying balloons, 3—inflow port, 4—outflow port, and 5—guidewire
2.4. Study procedure
Interventions were performed mainly by the contralateral femoral approach and with the use of 6F sheaths. Prior to treatment, atherectomy and pre‐dilatation of the target lesion with standard balloon(s) were performed before paclitaxel was delivered using the OPC. Radiopaque rulers were used to ensure that the zone treated with the OPC consistently exceeded the area treated with atherectomy and standard balloons by at least 10 mm. If more than one OPC treatment was used per lesion, the overlap zone was at least 10 mm. Once placement of the OPC was confirmed using the radiopaque markers, an inflation device was utilized to inflate the occlusion balloons using a mixture of contrast and saline to visualize and to define the treatment chamber. Varying contrast agents used in this study included Isovue 320 and 370 (Bracco, Monroe Township, NJ), Visipaque 270 and 320 (GE Healthcare, Chicago, IL), Omnipaque 350 (GE Healthcare, Chicago, IL), and Ultravist 300 (Bayer, Whippany, NJ). Paclitaxel diluted in contrast agent and saline at a concentration of 1.2 mg/ml was delivered, using an inflation device, into the treatment chamber through the inflow port. The column of the paclitaxel mixture filling the vessel was observed under fluoroscopy (Figure 2). Once blood exits the outflow port, the outflow port was closed, and the paclitaxel mixture was maintained in the treatment chamber under pressure for 2 min as measured by the pressure monitor attached to the OPC. Following the 2‐min dwell time, the paclitaxel was aspirated using an inflation device, the outflow port opened, and the treatment chamber flushed with saline to minimize systemic release of paclitaxel. The occlusion balloons were then deflated and the catheter was either removed safely from the patient or re‐positioned to deliver another dose of paclitaxel.
Figure 2.
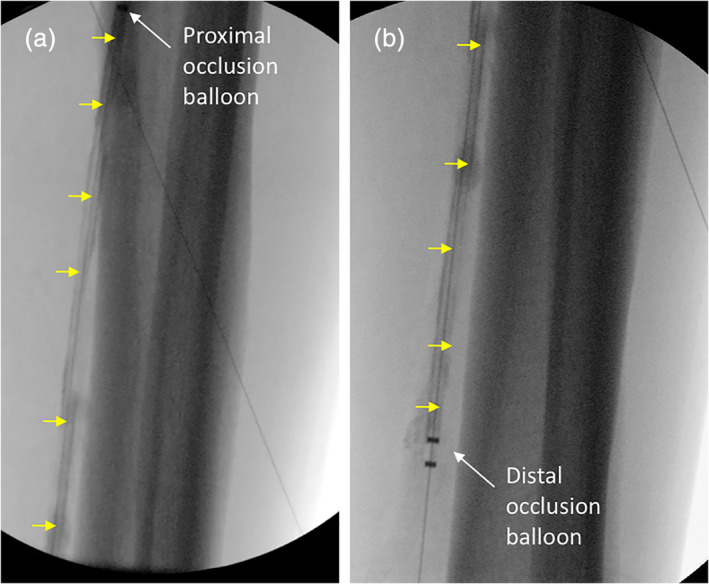
Angiographic image of the catheter during delivery. (a) Proximal portion of the occlusion perfusion catheter (OPC). White arrow indicates the proximal occlusion balloon and the yellow arrows show the treatment chamber filled with paclitaxel‐contrast mixture. (b) Distal portion of the OPC catheter. White arrow indicates the distal occlusion balloon and the yellow arrows show the treatment chamber filled with paclitaxel‐contrast mixture
All patients were taking 81 mg of aspirin daily. Post‐procedural medical therapy included aspirin 81 mg/day and clopidogrel 75 mg/day for a minimum of 4 weeks and aspirin therapy was continued with a dose of 81 mg/day thereafter.
2.5. Follow‐up, study end points and definitions
Follow‐up for each patient occurred at 1, 3, and 6 months. The primary efficacy endpoint in this study was patency, defined as freedom from target lesion occlusion, as determined by the ultrasound core laboratory and freedom from clinically driven target lesion revascularization (CD‐TLR) at 6 months. CD‐TLR was defined as lack of any TLR associated with deterioration of Rutherford classification and/or increasing size of pre‐existing wounds and/or occurrence of new wounds. Analysis of the TLR was done using the Kaplan–Meier survival analysis method. The primary safety endpoint in this study was freedom from major adverse events (MAEs) at 1 month, defined as TLR within 1 month, major amputation in the target limb (amputation above the metatarsals), and target limb related death. Secondary efficacy endpoints included device success defined as the ability to deliver paclitaxel to the interventional treatment area as intended, improvement in Rutherford category, Walking Impairment Questionnaire Scores at 6 months compared to baseline, and freedom from target vessel revascularization. Secondary safety endpoints were MAEs defined as target limb related death or major amputation in the target limb (amputation above the metatarsals) within the 3‐ and 6‐months post‐procedure.
2.6. Statistical analysis
Continuous data are presented as mean ± standard deviation. No comparative statistical analyses were performed; however, primary patency and target lesion revascularization rates were analyzed using Kaplan–Meier method.
3. RESULTS
3.1. Procedural outcomes
There were 35 patients (n = 35) enrolled at 11 sites in this study. The demographic data of the 35 patients are listed in Table 1. The average pre‐procedural diameter stenosis was 93.25% ± 8.94% with an average lesion length of 112 ± 81.2 mm. Reference vessel diameter was 3.2 ± 0.52 mm and 14 patients (39%) had a total occlusion. All patients were treated with a combination of atherectomy and balloon angioplasty prior to treatment with the OPC. Specifically, 14 patients were treated with laser atherectomy (Turbo‐Elite, Spectranetics, Colorado Springs, CO), 9 patients with directional atherectomy (Phoenix, Philips, Phoenix AZ & HawkOne, Medtronic, Minneapolis, MN), 3 patients with orbital atherectomy (Stealth 360, CSI, St. Paul, MN), and 9 patients with rotational atherectomy (JetStream & RotoBlator, Boston Scientific, Marlborough, MA). Debulking in all patients was technically successful with a final mean diameter stenosis of 5.7% ± 7.6%. Lesion characterization and their location are presented in Table 2.
Table 1.
Baseline patient characteristics
| Variable | (n = 35) |
|---|---|
| Demographic | |
| Age, years | 71 ± 9 |
| 72 [51–86] | |
| Men, n (%) | 28 (80) |
| Ethnicity, n (%) | |
| Caucasian/non‐Hispanic | 33 (94) |
| African‐American | 1 (3) |
| Other | 1 (3) |
| Body mass index, kg/m2 | 28.2 ± 5.6 |
| 28.3 [18.5–40.9] | |
| Clinical presentation | |
| Rutherford class, n (%) | |
| 2 | 1 (3) |
| 3 | 16 (45) |
| 4 | 14 (40) |
| 5 | 4 (12) |
| Ankle‐brachial index | 1.0 ± 0.38 |
| 1.0 [0.18–2.72] | |
| Medical history, n (%) | |
| Smoking history | 22/35 (63) |
| Current smoker | 6/22 (27) |
| Hypertension | 35 (100) |
| Hyperlipidemia | 34 (97) |
| Diabetes mellitus | 19 (55) |
| Obesity | 11 (33) |
| Myocardial infarction | 16/26 (62) |
| Previous coronary revascularization | 22/26 (85) |
Table 2.
Baseline lesion characteristics
| Variable | (n = 35) |
|---|---|
| Lesion type, n (%) | |
| De novo | 29 (81) |
| Restenotic | 7 (19) |
| Lesion location, n (%) | |
| Anterior Tibial (AT) | 17 (47) |
| Posterior Tibial (PT) | 9 (25) |
| Peroneal | 6 (17) |
| Popliteal/tibio‐peroneal trunk | 1 (3) |
| Tibio‐peroneal trunk | 1 (3) |
| Tibio‐peroneal trunk and peroneal | 2 (6) |
| Lesion geometry | |
| Lesion length, mm | 112 ± 81.2 |
| 97.5 [20–286] | |
| Reference vessel diameter, mm (per site) | 3.2 ± 0.52 |
| 3.0 [2.0–4.0] | |
| Diameter stenosis, (%) (per site) | 93.25 ± 8.94 |
| 99.00 [70–100] | |
| Total occlusion, n (%) | 14 (39) |
Device success, defined as the ability to deliver liquid paclitaxel to the interventional treatment area as intended was successful in 35 of 35 (100%) lesions. All patients were treated with the 3.0 mm × 150 mm OPC catheter for 2 min. In 16 out of the 35 lesions treated, a pull‐back procedure was used to deliver paclitaxel to lesions >130 mm in length. This procedure starts with treating the most distal region of the lesion followed by more proximal segment with an overlap area of 10–20 mm. No thrombosis and only one Grade A dissection (3%) of the treated lesions were identified during the final angiographic images following delivery of paclitaxel by the OPC with zero bailout stent placement. The observed Grade A dissection was observed following atherectomy and pre‐dilatation of the target lesion, prior to OPC placement. Table 3 summarizes the procedural data for all treated patients. An example of pre‐ and post‐angiogram of a treated lesion is shown in Figure 3.
Table 3.
Procedural data
| Variable | (n = 35) |
|---|---|
| Total procedure time, minutes | 113 ± 44 |
| 103 [48–217] | |
| OPC placement, minutes | 14 ± 09 |
| 11 [04–48] | |
| Procedure success, n (%) | 35 (100) |
| Atherectomy performed, n (%) | 35 (100) |
| Laser | 14 (40) |
| Directional | 9 (26) |
| Orbital | 3 (9) |
| Rotational | 9 (26) |
| Treatment chamber pressure, atm | |
| Baseline | 0.18 ± 0.10 |
| 0.16 [0.04–0.53] | |
| 1 min | 0.17 ± 0.08 |
| 0.15 [0.04–0.51] | |
| 2 min | 0.16 ± 0.07 |
| 0.14 [0.04–0.50] | |
| Indeflator delivery pressure, atm | |
| Baseline | 3.5 ± 2.4 |
| 2.7 [1.0–14.0] | |
| 1 min | 2.9 ± 2.3 |
| 2.0 [0.5–14.0] | |
| 2 min | 2.6 ± 2.5 |
| 2.0 [1.0–14.0] | |
| Dissection, n (%) (pre‐OPC placement) | |
| None | 34 (97) |
| Grade A | 1 (3) |
| Grade B | 0 (0) |
| Grade C | 0 (0) |
| Grade D | 0 (0) |
| Dissection, n (%) (post‐OPC placement) | |
| None | 35 (100) |
| OPC treatments, n (%) | |
| Single | 19 (54) |
| 2–3× | 16 (46) |
| 4–5× | 0 (0) |
| 6–8× | 0 (0) |
| Paclitaxel dose | |
| Per patient (mg/m2) | 4.27 ± 3.0 |
| 3.6 [0.7–15.5] | |
| Per placement (mg) | 6.2 ± 4.6 |
| 4.2 [1.2–19.2] | |
| Bailout stent placement, n (%) | 0 (0) |
| Diameter stenosis after the intervention; pre OPC | 5.7 ± 7.6 |
| 0.0 [0–25] | |
Abbreviation: OPC, occlusion perfusion catheter.
Figure 3.
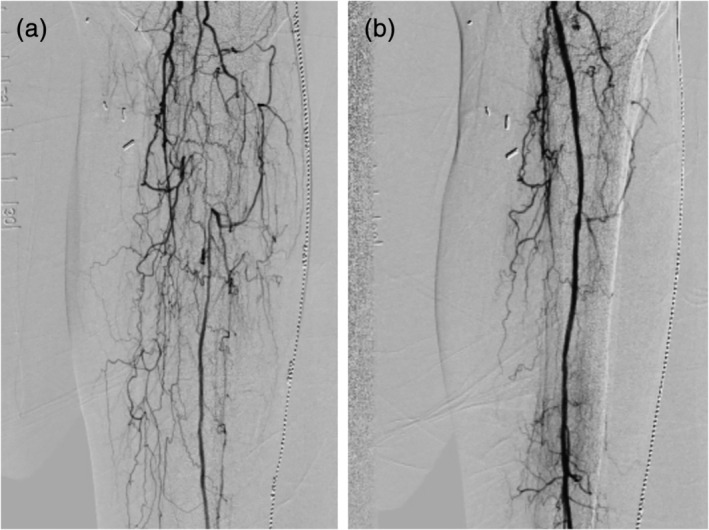
Angiogram examples of vessel occlusion before and after treatment. (a) The peroneal and anterior tibial vessels were pre‐treated with atherectomy and balloon angioplasty, followed by 2 min of drug delivery by the occlusion perfusion catheter. (b) Results show excellent flow with minimal residual stenosis after localized delivery of paclitaxel
3.2. Safety and efficacy outcomes
Through the 6‐month follow‐up, there were two deaths, unrelated to the procedure, one patient withdrawal and four patients lost to follow‐up. The two unrelated deaths were due to acute myocardial infarction and coronary artery disease with diabetic complications. There were no incidences of target limb related thrombosis or major amputations (Table 4). Primary patency, as determined by Duplex Doppler Ultrasound, was 89.3% (25 of 28 patients). Freedom from clinically driven target lesion revascularization was 96.4% (27 of 28 patients). Rutherford classification of patients was 3.6 ± 0.7 at pre‐treatment, 1.4 ± 1.2 at 1‐month post‐treatment, and 1.8 ± 1.1 at 6‐month post‐treatment. There were no reports of device or procedure related serious adverse events through 6‐months post‐procedure.
Table 4.
Efficacy, safety, and functional outcomes
| Variable | (n = 35) |
|---|---|
| 6‐month primary efficacy outcome | |
| Freedom from CD‐TLR, n (%) | 27/28 (96.4) |
| Primary patency, n (%) | 25/28 (89.3) |
| 6‐month primary safety outcome | |
| Death, n (%) | 2 (5.7) |
| Thrombosis, n (%) | 0 |
| Major amputation in target limb (%) | 0 |
| Ankle‐brachial index | |
| Screening | 1.0 ± 0.38 |
| 1.0 [0.18–2.72] | |
| 3 months | 1.1 ± 0.21 |
| 1.1 [0.66–1.57] | |
| 6 months | 1.0 ± 0.28 |
| 1.1 [0.48–1.50] | |
| Rutherford score | |
| Screening | 3.6 ± 0.7 |
| 4 [2–5] | |
| 3 months | 1.4 ± 1.2 |
| 1 [0–4] | |
| 6 months | 1.8 ± 1.1 |
| 2 [0–4] | |
Abbreviation: CD‐TLR, clinically driven target lesion revascularization.
4. DISCUSSION
This multicenter study clinically demonstrates the safety and efficacy of liquid paclitaxel administered using the OPC delivery catheter for the prevention of restenosis in infrapopliteal de novo and restenotic lesions. Although our previous first‐in‐human study found encouraging safety and initial feasibility, this more elaborate study provides further evidence into this novel approach with larger number of patients and more stringent evaluation of the treated lesion.13 The primary safety outcome demonstrated no treatment related deaths, thrombosis or major amputation. Primary patency was 89.3% and freedom from clinically driven target lesion revascularization rate was 96.4% in lesions treated with a mean length of 119.1 ± 81.2 mm. Together, these results provide encouragement in the interventional treatment of the heavy atherosclerotic disease burden in BTK arteries and provide further insight into the local liquid therapy approach.
Treatment of BTK disease remains a major hurdle in endovascular therapy; in particular, as the majority of these patients suffer from CLI with increased risk of cardiovascular disease. Historically, balloon angioplasty provided poor patency outcomes in these vessels with restenosis rates of 35% at 6 months and increasing to 46%–58% at 1 year.14, 15, 16 The use of DCBs was seen as a better alternative to balloon angioplasty, in particular for diffuse and long lesions of the periphery. However, the benefit of DCB to treat BTK disease remains controversial, primarily due to safety concerns.9, 10, 12 The randomized controlled trial studying the IN.PACT Amphirion DCB demonstrated a 6‐month primary safety endpoint of the DCB arm was 17.7%, and the 12‐month amputation and death was reported as 35.2%. A trend toward an increased major amputation and death rate was shown in the DCB arm.9 Although the reported safety of DCB for BTK arteries have yet to be determined, adverse events associated with DCB has been suggested to correlate with distal coating embolization as shown in pre‐clinical animal studies.17 More recently, a systematic review and meta‐analysis of randomized controlled trials investigating paclitaxel‐coated devices for peripheral applications showed an increased risk of death.18 The authors postulate late paclitaxel toxicity, associated with crystallinity of paclitaxel coating, may be the reason for the observed increased in death rate.18 Additionally, the long‐term impact of excipients on paclitaxel safety in DCBs remain relatively unknown.
The OPC device delivers a combination of liquid paclitaxel, contrast agent (serving as the excipient) and saline as the liquid therapeutic agent to inhibit neointimal growth. The intravenous (liquid) paclitaxel delivered by the OPC device is designed with a half‐life of around 6 hr,19, 20 whereas the crystallin paclitaxel in DCBs are specifically designed not to break down and remain insoluble, having a half‐life of weeks to months.21, 22, 23 The intravascular (liquid) paclitaxel has been utilized for decades primarily in patients with ovarian and metastatic breast cancer, with cancer patients receiving roughly 300 mg per treatment (one order magnitude higher than patients treated with the OPC catheter). The intravascular liquid paclitaxel is designed as a highly soluble drug, increasing the uptake of the drug by cells. On the other side of the spectrum, crystallin paclitaxel has very poor solubilization properties. Although the crystallinity of the drug increases local arterial pharmacokinetic (long‐term arterial tissue paclitaxel is increased), any lost paclitaxel coating during transit and deployment can potentially remain and accumulate within distal tissue and organs. Preclinical studies have shown ~1%–10% of the paclitaxel coated on DCBs get transferred into the arterial wall, with the remaining (up to 90%) lost into the circulation.22, 24 Recent pre‐clinical publications has shown DCB coating particulates (lost into circulation) lead to fibrinoid necrosis within downstream skeletal muscle tissue.17, 25
The fundamental delivery approach of the OPC also differs from DCBs. In liquid delivery using the OPC, pressure in the treatment chamber uniformly delivers liquid paclitaxel into the vessel wall regardless of the cross‐sectional shape of the vessel. In DCB however, successful delivery of drug coated on the surface of the balloon is highly dependent on the cross‐sectional shape of the vessel, with a preference toward a circular cross‐sectional area to maximize balloon‐to‐artery contact area. DCB places finite paclitaxel deposits onto the luminal surface, with the goal that these paclitaxel deposits will solubilize and diffuse into the vessel wall. The OPC device directly delivers liquid paclitaxel into the arterial wall following the two‐minute delivery time during treatment as previously shown.26
Overall the use of liquid oncological paclitaxel reduces safety concerns associated with crystalline paclitaxel form and long‐term paclitaxel toxicity. Additionally, the design of the OPC system ensures little to no loss of paclitaxel during tracking of the device to the lesion as the drug is not infused at the treatment location until the treatment chamber has been established by inflation of the occlusion balloons (Figure 2). The absence of drug coatings and the use of liquid paclitaxel also minimize the risk of embolization as observed in DCB pre‐clinical studies.17, 25 Lastly, in comparison to a DCB, there is minimal barotrauma during drug delivery by the OPC as balloon sizing is not a factor, in particular, for longer and tapered vessels, reducing the risks of dissection during drug delivery.
The reported primary patency of 89.3% and freedom from clinically driven target lesion revascularization rate of 96.4% in the OPC‐treated lesions are very encouraging. Although our study was not designed to compare with previous studies due to our smaller cohort, our reported freedom from CD‐TLR of 96.4% was numerically higher than the reported Lutonix 014 DCB global BTK registry study27 (6‐month follow‐up, 87.9%), a single‐center Lutonix DCB study11 (9‐month follow‐up, 84.1% [6‐month follow‐up not reported]), DEBELLUM28(6‐month follow‐up, 93.9%), DEBATE BTK2 (12‐month follow‐up, 81.5% [6‐month follow‐up not reported]), IN.PACT DEEP9 (12‐month follow‐up, 90.8% [6‐month follow‐up not reported]), IDEAS29 (6 month follow‐up, 86.4%), and BIOLUX P‐II10 (6 month follow‐up, 80%) clinical studies. We contribute the success of this trial to the design of the OPC, in which drug is delivered directly to the medial layer using pressure. The built‐in OPC pressure sensor, which continuously monitors the chamber pressure during delivery, enables a quantifiable manner to ensure consistent and appropriate liquid paclitaxel delivery by the operator. This is an essential feature that has not been available in previous generation of liquid delivery devices.
The average treatment chamber pressure was 0.16 ± 0.07 atm whereas the indeflator pressure was 2.6 ± 2.5 atm. Figure 4 shows the variation in the indeflator pressure versus the treatment chamber pressure for this study. It can be observed that no direct correlation exists between indeflator pressure and the treatment chamber pressure. To achieve the desired treatment chamber, indeflator pressure can vary from 1.0 to 14.0 atm. This large variation is likely due to varying vessel compliance, disease severity, branching, viscosity of the therapeutic agent, and artery debulking. It is therefore vital in local liquid delivery approach to monitor in real time treatment chamber pressure to ensure adequate treatment chamber pressure while minimizing drug volume delivery and barotrauma.
Figure 4.
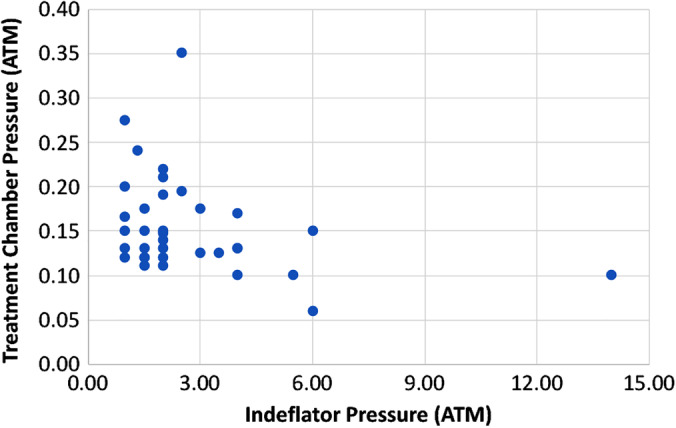
Indeflator pressure versus treatment chamber pressure for all patients
This current study shows the potential benefit of such a universal catheter to treat BTK arterial revascularization, however, the study does suffer from certain limitations. Long term outcomes are not known as the COPPER‐A BTK study included a small number of patients with a 6‐month follow‐up. As this was a feasibility and safety analysis, this study included patients ranging from claudication to critical limb ischemia. While most patients had CLI (51.4%), future studies will need to investigate its role in Rutherford six patients. Furthermore, while the OPC device can be utilized to treat multiple BTK vessels of varying lengths, the safety and feasibility of this treatment approach will need to be investigated as patients in this cohort had one vessel treated with drug delivery. Finally, all patients were treated with atherectomy. The role of debulking heavily diseased BTK vessels and its effect on local liquid paclitaxel pharmacokinetics and subsequent patency/TLR will need to be further elucidated in future research. The study also lacked DCB and balloon angioplasty comparative groups. Future studies should include angiographic follow‐up to assess binary restenosis at the treatment site and wound care assessment.
5. CONCLUSION
Treatment of infrapopliteal arteries remains unresolved and associated with a high restenosis rates and poor long‐term clinical patency. The results from this multi‐center study shows the potential of the OPC catheter to safely and effectively treat de novo and restenotic BTK lesions. The delivery of liquid paclitaxel using the OPC catheter under controlled pressure was technically achievable without procedural complications. These results provide encouragement for local liquid delivery as an alternative approach for infrapopliteal revascularization. In particular, the ability to treat very long or multiple lesions with a single device, provides a more economical option. The safety profile in this study is particularly favorable in view of recent concerns regarding adverse events with crystalline‐paclitaxel coated devices. Longer term follow‐up and larger clinical studies will be needed to support our findings of this technology with head‐to‐head comparisons with DCB and balloon angioplasty.
ACKNOWLEDGMENTS
The authors would like to thank Eminence Clinical Research, Inc. (Colorado Springs, CO) and Saami Yazdani PhD for statistical support and medical writing assistance.
Bunch F, Nair P, Aggarwala G, et al. A universal drug delivery catheter for the treatment of infrapopliteal arterial disease using liquid therapy. Catheter Cardiovasc Interv. 2020;96:393–401. 10.1002/ccd.28739
EDITORIAL COMMENT: Expert Article Analysis for: Locally applied chemotherapy is where it's at: New hope in treating infrapopliteal disease
REFERENCES
- 1. Hirsch AT, Duval S. Effective vascular therapeutics for critical limb ischemia: a role for registry‐based clinical investigation. Circ Cardiovasc Interv. 2013;6(1):8‐11. 10.1161/CIRCINTERVENTIONS.113.000127. [DOI] [PubMed] [Google Scholar]
- 2. Liistro F, Porto I, Angioli P, Grotti S, Ricci L, Ducci K, Falsini G, Ventoruzzo G, Turini F, Bellandi G and others. Drug‐eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE‐BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation 2013;128(6):615–621. [DOI] [PubMed] [Google Scholar]
- 3. Clair D, Shah S, Weber J. Current state of diagnosis and management of critical limb ischemia. Curr Cardiol Rep. 2012;14(2):160‐170. 10.1007/s11886-012-0251-4. [DOI] [PubMed] [Google Scholar]
- 4. Faglia E, Clerici G, Clerissi J, Gabrielli L, Losa S, Mantero M, Caminiti M, Curci V, Quarantiello A, Lupattelli T and others. Long‐term prognosis of diabetic patients with critical limb ischemia: a population‐based cohort study. Diabetes Care 2009;32(5):822–827. doi: 10.2337/dc08-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fabiani I, Calogero E, Pugliese NR, Di Stefano R, Nicastro I, Buttitta F, Nuti M, Violo C, Giannini D, Morgantini A and others. Critical limb ischemia: a practical up‐to‐date review. Angiology 2018;69(6):465–474. [DOI] [PubMed] [Google Scholar]
- 6. Adam DJ, Bradbury AW. TASC II document on the management of peripheral arterial disease. Eur J Vasc Endovasc Surg. 2007;33(1):1‐2. [DOI] [PubMed] [Google Scholar]
- 7. Duda SH, Poerner TC, Wiesinger B, et al. Drug‐eluting stents: potential applications for peripheral arterial occlusive disease. J Vasc Interv Radiol. 2003;14(3):291‐301. [DOI] [PubMed] [Google Scholar]
- 8. Scheinert D, Duda S, Zeller T, et al. The LEVANT I (Lutonix paclitaxel‐coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first‐in‐human randomized trial of low‐dose drug‐coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv. 2014;7(1):10‐19. [DOI] [PubMed] [Google Scholar]
- 9. Zeller T, Baumgartner I, Scheinert D, et al. Drug‐eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12‐month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol. 2014;64(15):1568‐1576. 10.1016/j.jacc.2014.06.1198. [DOI] [PubMed] [Google Scholar]
- 10. Zeller T, Beschorner U, Pilger E, et al. Paclitaxel‐coated balloon in Infrapopliteal arteries: 12‐month results from the BIOLUX P‐II randomized trial (BIOTRONIK'S‐first in man study of the Passeo‐18 LUX drug releasing PTA balloon catheter vs. the uncoated Passeo‐18 PTA balloon catheter in subjects requiring revascularization of infrapopliteal arteries). JACC Cardiovasc Interv. 2015;8(12):1614‐1622. 10.1016/j.jcin.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 11. Steiner S, Schmidt A, Bausback Y, et al. Single‐center experience with Lutonix drug‐coated balloons in Infrapopliteal arteries. J Endovasc Ther. 2016;23(3):417‐423. [DOI] [PubMed] [Google Scholar]
- 12. Kayssi A, Al‐Atassi T, Oreopoulos G, Roche‐Nagle G, Tan KT, Rajan DK. Drug‐eluting balloon angioplasty versus uncoated balloon angioplasty for peripheral arterial disease of the lower limbs. Cochrane Database Syst Rev. 2016;8:CD011319 10.1002/14651858.CD011319.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bunch F, Walker C, Kassab E, Carr J. A universal drug delivery catheter for the treatment of infrapopliteal arterial disease: results from the multi‐center first‐in‐human study. Catheter Cardiovasc Interv. 2018;91(2):296‐301. [DOI] [PubMed] [Google Scholar]
- 14. Schamp KB, Meerwaldt R, Reijnen MM, Geelkerken RH, Zeebregts CJ. The ongoing battle between infrapopliteal angioplasty and bypass surgery for critical limb ischemia. Ann Vasc Surg. 2012;26(8):1145‐1153. 10.1016/j.avsg.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 15. Romiti M, Albers M, Brochado‐Neto FC, Durazzo AE, Pereira CA, De Luccia N. Meta‐analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47(5):975‐981. [DOI] [PubMed] [Google Scholar]
- 16. Conte MS, Geraghty PJ, Bradbury AW, et al. Suggested objective performance goals and clinical trial design for evaluating catheter‐based treatment of critical limb ischemia. J Vasc Surg. 2009;50(6):1462‐1473. e1‐3. [DOI] [PubMed] [Google Scholar]
- 17. Kolodgie FD, Pacheco E, Yahagi K, Mori H, Ladich E, Virmani R. Comparison of particulate embolization after femoral artery treatment with IN.PACT admiral versus Lutonix 035 paclitaxel‐coated balloons in healthy swine. J Vasc Interv Radiol. 2016;27(11):1676‐1685. [DOI] [PubMed] [Google Scholar]
- 18. Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel‐coated balloons and stents in the Femoropopliteal artery of the leg: a systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2018;7(24):e011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004‐1014. [DOI] [PubMed] [Google Scholar]
- 20. Sonnichsen DS, Relling MV. Clinical pharmacokinetics of paclitaxel. Clin Pharmacokinet. 1994;27(4):256‐269. [DOI] [PubMed] [Google Scholar]
- 21. Gongora CA, Shibuya M, Wessler JD, et al. Impact of paclitaxel dose on tissue pharmacokinetics and vascular healing: a comparative drug‐coated balloon study in the familial hypercholesterolemic swine model of superficial femoral in‐stent restenosis. JACC Cardiovasc Interv. 2015;8(8):1115‐1123. [DOI] [PubMed] [Google Scholar]
- 22. Speck U, Cremers B, Kelsch B, et al. Do pharmacokinetics explain persistent restenosis inhibition by a single dose of paclitaxel? Circ Cardiovasc Interv. 2012;5(3):392‐400. [DOI] [PubMed] [Google Scholar]
- 23. Granada JF, Stenoien M, Buszman PP, et al. Mechanisms of tissue uptake and retention of paclitaxel‐coated balloons: impact on neointimal proliferation and healing. Open Heart. 2014;1(1):e000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stolzenburg N, Breinl J, Bienek S, et al. Paclitaxel‐coated balloons: investigation of drug transfer in healthy and atherosclerotic arteries – first experimental results in rabbits at low inflation pressure. Cardiovasc Drugs Ther. 2016;30(3):263‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torii S, Yahagi K, Mori H, et al. Biologic drug effect and particulate embolization of drug‐eluting stents versus drug‐coated balloons in healthy swine femoropopliteal arteries. J Vasc Interv Radiol. 2018;29(7):1041‐1049. e3. [DOI] [PubMed] [Google Scholar]
- 26. Atigh MK, Turner E, Christians U, Yazdani SK. The use of an occlusion perfusion catheter to deliver paclitaxel to the arterial wall. Cardiovasc Ther. 2017;35(4):e12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thieme M, Lichtenberg M, Brodmann M, Cioppa A, Scheinert D. Lutonix(R) 014 DCB global below the knee registry study: interim 6‐month outcomes. J Cardiovasc Surg (Torino). 2018;59(2):232‐236. [DOI] [PubMed] [Google Scholar]
- 28. Fanelli F, Cannavale A, Boatta E, et al. Lower limb multilevel treatment with drug‐eluting balloons: 6‐month results from the DEBELLUM randomized trial. J Endovasc Ther. 2012;19(5):571‐580. [DOI] [PubMed] [Google Scholar]
- 29. Siablis D, Kitrou PM, Spiliopoulos S, Katsanos K, Karnabatidis D. Paclitaxel‐coated balloon angioplasty versus drug‐eluting stenting for the treatment of infrapopliteal long‐segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC Cardiovasc Interv. 2014;7(9):1048‐1056. 10.1016/j.jcin.2014.04.015. [DOI] [PubMed] [Google Scholar]


