Figure 1.
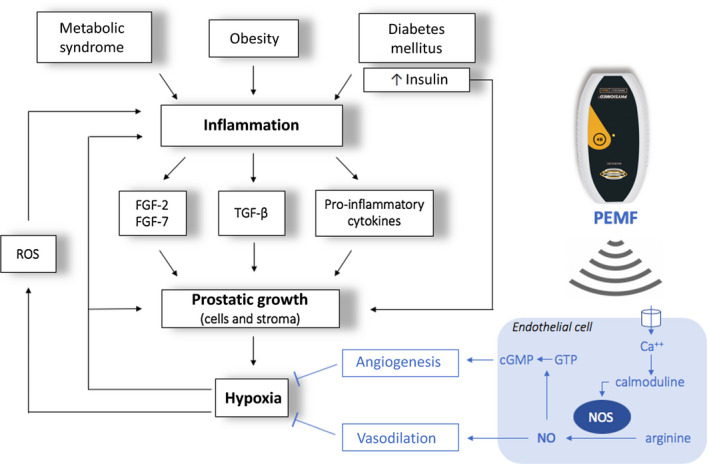
Inflammatory hypothesis underlying BPH pathogenesis and biophysical mechanism of PEMF efficacy: during inflammation mitogen substances such as cytokines (IL‐2, IL‐4, IL‐8, IL‐15, IL‐17, IFN‐γ) and growth factors (VEGF, TGF‐β, FGF‐2, FGF‐7) are released, resulting in an abnormal proliferation of prostatic cells and stroma. FGFs primarily stimulate fibroblasts to produce fibromuscular tissue and also stimulate angiogenesis, proliferation, and differentiation of stromal and epithelial prostatic cells. The TGF‐β stimulates the differentiation of smooth muscle cells and the development of abundant extracellular matrix. The overproduction of stroma strongly increases oxygen consumption, and it is therefore responsible for hypoxia in the transition zone of the prostate. Hypoxia itself activates several signaling pathways that regulate angiogenesis and tissue proliferation. Furthermore, local hypoxia promotes the release of reactive oxygen species (ROS) that, in turn, stimulate the release of growth factors (IL‐8, FGF‐7, TGF‐β, FGF‐2) and consequent glandular hyperplasia. The biophysical mechanism of PEMF efficacy is likely to involve an electrochemical model of the cell membrane: PEMF seems to increase intracellular calcium (Ca2+) binding to calmoduline. This bond activates the calmoduline pathway which catalyzed endothelial nitric oxide synthase isoform (eNOS), an enzyme responsible for the synthesis of nitric oxide (NO) and citrulline from L‐arginine and O2. NO activates an anti‐inflammatory response by recalling lymphocytes from the blood, and it also causes vasodilation with a consequent increase in local blood flow and reduction of hypoxia. Furthermore, NO regulates cGMP signaling cascades that promote angiogenesis and tissue remodeling. The overall effect is a reduction in tissue hypoxia and therefore a reduction in prostatic growth.6, 12, 16, 17
