Figure 1.
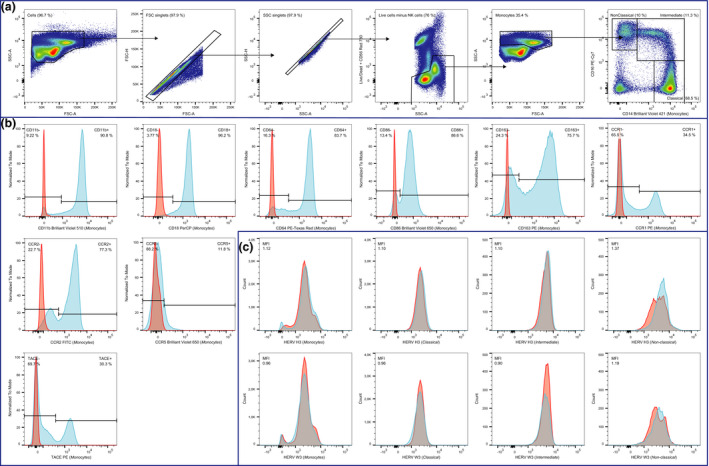
Gating strategy used in the flow cytometric analysis. A sample from a representative patient with RRMS was used for this figure. (a) From left to right: Total PBMCs (> 300,000 events) were gated according to their size and granularity in forward scatter‐area (FSC‐A) / side scatter‐area (SSC‐A); aggregated cells were removed according to forward scatter‐area (FSC‐A) / FSC‐H and side scatter‐area (SSC‐A) / SSC‐H leaving only singlets; dead cells as well as NK‐cells were removed according to staining with a LIVE/DEAD cell stain and monoclonal anti‐CD56 antibody, respectively; monocytes (> 100,000 events) were gated in forward scatter‐area (FSC‐A) / side scatter‐area (SSC‐A); and finally the three monocyte subsets (classical, intermediate, non‐classical) were gated according to their CD14/CD16 expression. (b) From left to right: Surface marker expression of CD11b, CD18, CD40, CD64, CD86, CD163, CCR1, CCR2, CCR5, and TACE were determined by gating on positive cells (blue). Appropriate isotype controls (red) were used to determine the unspecific antibody binding. (c) From left to right: Human endogenous retrovirus (HERV) expression was determined on the monocyte population, and on the three monocyte subsets (classical, intermediate, non‐classical) by incubation with sera from rabbits immunized with HERV H3 Env (top row) or HERV W3 Env (bottom row) peptide antigens (blue) as described previously 36 and with the appropriate control (pre‐immune sera) (red) to determine the positive populations (shaded grey area represent the overlap between the two curves).
