Figure 2.
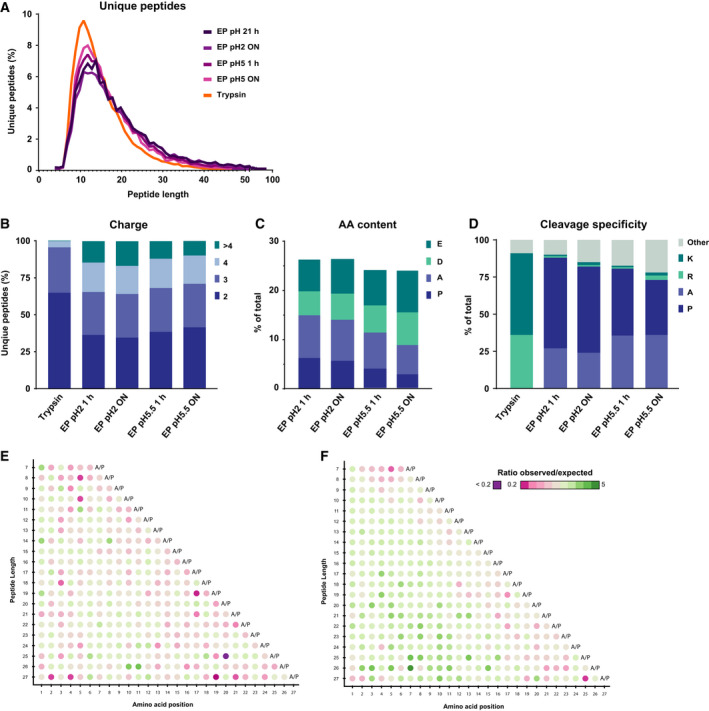
Comparison of peptide characteristics in EndoPro and tryptic digests. (A) Peptide length distribution of identified unique peptides following digestion with trypsin or EndoPro. All four EndoPro conditions probed here reveal a similar distribution, exhibiting a long tail toward peptides with more than 50 amino acids, which was not observed for tryptic peptides. (B) Charge distribution of all unique peptides identified following the different digestion conditions, where digestion with EndoPro results in more highly charged peptides (z ≥ 4). (C) Amino acid content of the peptides identified in the EndoPro digests under various digestion conditions. With increase in pH and digestion duration, negatively charged amino acids are more frequently observed and the A/P content of the peptides is reduced. (D) Cleavage specificity of the identified peptides. Digestion with EndoPro yields highly specific proline and alanine C‐terminal peptides, especially at pH = 2, with a Pro/Ala specificity close to that of trypsin for Arg/Lys. (E, F) Location of Asp on peptides digested ON with EndoPro at (E) pH = 2 and (F) pH = 5.5. At pH = 5.5, the negatively charged amino acid is disfavored at the C terminus of the generated peptides. This was not observed for peptides produced at pH = 2, indicating that two distinct sets of peptides are formed at these pH values.
