Figure 5.
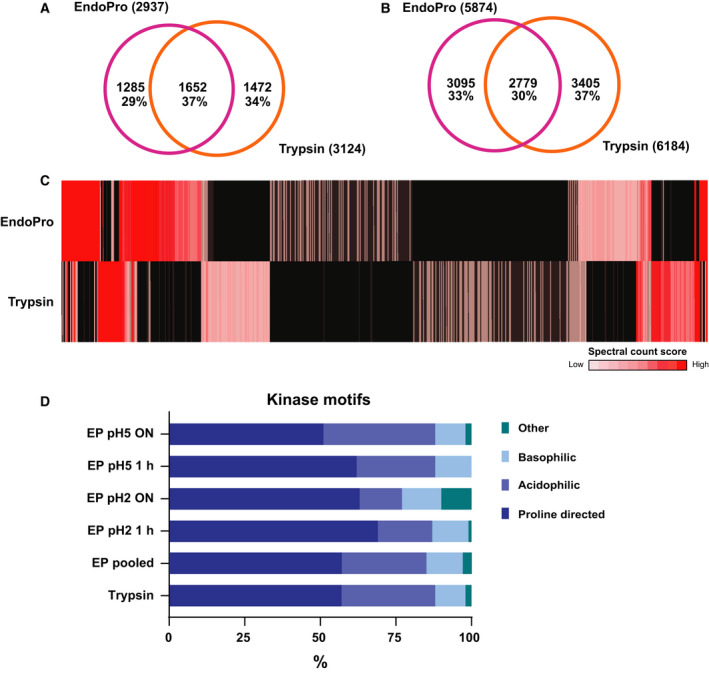
EndoPro is highly complementary to trypsin in the identification of site‐specific phosphorylation events. (A) Comparison of identified unique phosphoproteins between EndoPro and trypsin, revealing a 37% overlap. (B) Overlap in identified unique phosphosites on 1652 phosphoproteins identified by both proteases, indicating that on these shared phosphoproteins, only 30% of the phosphosites could be identified by both proteases. (C) Heatmap displaying phosphosite spectral count scores of 13 762 phosphosites from low (1) to high (> 10), revealing that EndoPro is highly complementary to trypsin in identification of phosphosites. Black indicated not identified. (D) Global kinase classification analysis of all identified phosphopeptides, dividing them into 4 categories: proline‐directed, acidophilic, basophilic, or other. Although in all analyses the SP/TP motif encompasses over 50% of the detected sites, short digestion with EndoPro results in a further increase of this proline‐directed motif to about 70%.
