Abstract
Waldenström's macroglobulinaemia (WM) is a rare indolent B‐cell lymphoma for which only little prospective phase III evidence exists. Thus, real world data are important to provide insight into treatment and survival. We present here data on choice and outcome of systemic treatment of patients with WM treated in German routine practice. In total, 139 patients with WM who had been documented in the prospective clinical cohort study Tumour Registry Lymphatic Neoplasms (NCT00889798) were included into this analysis. We analysed the most frequently used first‐line and second‐line treatments between 2009 and 2017 and examined best response, progression‐free survival (PFS) and overall survival (OS). Bendamustine plus rituximab, with a median of six cycles, was by far the most frequently used first‐line treatment (81%). Second‐line treatment was more heterogenous and mainly based on bendamustine, cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP), fludarabine or ibrutinib, the latter approved in 2014. Three‐year PFS from start of first‐line treatment was 83% (95% confidence interval [CI] 74%‐88%), 3‐year OS was 87% (95% CI 80%‐92%). These prospective data give valuable insights into the management and outcome of non‐selected patients with WM treated in German routine practice. In the lack of prospective phase III clinical trials, real world data can help bridging the gap of evidence.
Keywords: disease management; lymphoma, B‐cell; outpatients; prognosis; registries; Waldenström's macroglobulinaemia
1. INTRODUCTION
Waldenström's macroglobulinaemia (WM) is a rare indolent B‐cell lymphoma that accounts for 1% to 2% of non‐Hodgkin's lymphomas (NHLs). 1 It is a disease of the elderly, with a range of median age at diagnosis from 63 to 75 years. 1 WM is characterised by immunoglobulin M (IgM) monoclonal gammopathy and infiltration of the bone marrow by clonal lymphoplasmacytic cells.2, 3 Lately, whole genome sequencing has identified activating mutations in MYD88 present in more than 90% of patients with WM and mutations in CXCR4 in approximately 30% to 40% of patients.4, 5 The age‐adjusted incidence rate has been reported to be 5.5 per million European standard population using cancer registry data from the UK 6 ; in Germany, the estimated incidence rate is 3.8 per million.7, 8 Patients with WM can remain asymptomatic for years without requiring therapy. 9 Systemic treatment is usually initiated in patients who present with anaemia, hyperviscosity or other constitutional symptoms. 10 Choice of first‐line treatment is guided by the clinical presentation of the disease and/or complications 1 and individual patient's characteristics. 11 Since WM incidence is low, few phase III randomised clinical trials (RCTs) have been conducted so far.9, 10, 12, 13, 14, 15 Thus, treatment recommendations and individual decisions are mostly based on phase II data. 16 The lack of a clear standard of care for first‐line treatment of WM is reflected by the heterogeneity in the treatment approaches used.9, 16 The anti‐CD20 monoclonal antibody rituximab is the mainstay of most therapeutic regimens.1, 2 Rituximab is often combined with alkylating agents, such as bendamustine, chlorambucil and cyclophosphamide, or nucleoside analogues, such as fludarabine or proteasome inhibitors, such as bortezomib.1, 9, 10, 14, 17, 18, 19, 20 Recently, the irreversible Bruton's tyrosine kinase inhibitor ibrutinib has broadened the treatment options for patients with WM.10, 21, 22 The International Prognostic Scoring System for WM (ISSWM 23 ) based on patient and disease parameters evaluated at start of first‐line treatment (including age, haemoglobin, platelet counts, beta‐2 microglobulin and monoclonal IgM concentration) is the most widely established prognostic index which helps predict prognosis for patients with WM. 11 However, the current ISSWM is challenged by data analysing factors with additional impact on early mortality including non‐WM‐related mortality in specific age clusters (>75 years) 24 as well as LDH and serum albumin. 25 The latter are well‐established prognostic factors in other indolent lymphomas. Although overall survival (OS), WM‐related and non‐WM‐related mortality have improved over time, 26 WM—like other indolent lymphomas—remains incurable. 27
Owing to the scarce evidence from prospective phase III RCTs, routine data are of great importance to identify treatment and survival of patients with WM, particularly, when data have been prospectively collected. In this article, we present data on 139 patients with WM who all underwent systemic treatment. Data were collected within the prospective clinical cohort study TLN (Tumour Registry Lymphatic Neoplasms) which had recruited patients with indolent NHL28, 29, 30 or aggressive NHL 31 treated by office‐ and hospital‐based haematologists in Germany. We show the choice of first‐line treatment (2009‐2014) and second‐line treatment (2010‐2017) and the treatment outcome by analysing best response, progression‐free survival (PFS) and OS.
2. METHODS
2.1. Data source
The TLN is an open, longitudinal, multicentre, observational, prospective cohort study collecting data on the treatment of patients with lymphoid B‐cell neoplasms. The study started in 2009 and was approved by the responsible ethics committee; it is registered at ClinicalTrials.gov (NCT00889798). Patients aged ≥18 years with indolent or aggressive NHL at start of their first‐ or second‐line treatment were recruited into the TLN. Written informed consent was obtained from all patients. Treatment of patients started within 4 weeks prior or until 8 weeks after signing the informed consent. Patients were treated according to physicians' choice and visited their physician on their individual schedule. Patients were followed for up to 5 years from enrolment or until death, loss to follow‐up or withdrawal of consent. Further details on the methodology of the TLN have been previously described elsewhere.28, 29, 30, 31
2.2. Cohort definition
By the end of the enrolment period in August 2014, 3795 patients with lymphoid B‐cell neoplasms had been recruited into the TLN (Figure 1). Two hundred and ten patients were not evaluable due to incomplete data on treatment or withdrawal of consent. Out of the remaining 3585 evaluable patients, 1187 were diagnosed with indolent NHL (excluding chronic lymphocytic leukaemia and multiple myeloma); 1049 of them had been enrolled at start of their first‐line treatment. Among them, we identified 139 patients with WM who had been enrolled in 73 office‐ and hospital‐based medical oncology centres across Germany between April 2009 and January 2014. Data cut‐off for this analysis was 31 August 2018.
FIGURE 1.
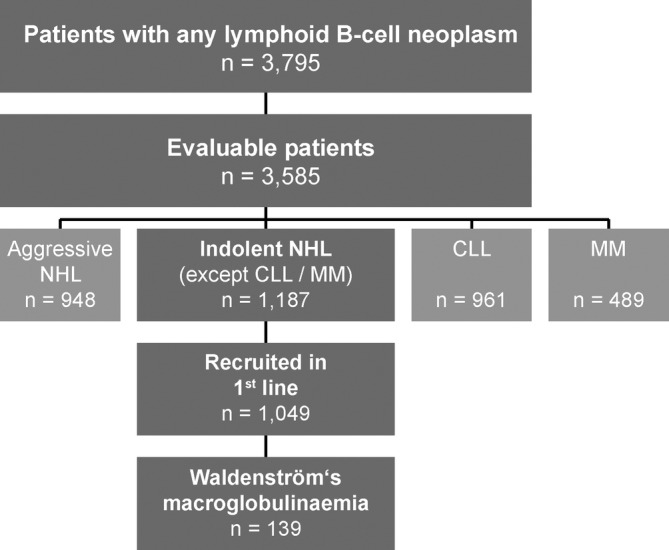
Cohort definition. Number of patients enrolled in the TLN from April 2009 until August 2014, split up according to different types of lymphoid B‐cell neoplasms. Of all evaluable patients with indolent NHL (other than CLL or MM), those patients with WM who had been prospectively enrolled at the start of their first‐line treatment were included into this analysis (n = 139). Data cut‐off for this analysis was 31 August 2018. CLL, chronic lymphocytic leukemia; MM, multiple myeloma; NHL, non‐Hodgkin's lymphoma; TLN, Tumour Registry Lymphatic Neoplasms; WM, Waldenström's macroglobulinaemia
2.3. Statistical analysis
Time‐to‐events was analysed by the Kaplan‐Meier method. PFS was defined as the interval between start of first‐line treatment and date of progression or death prior to start of second‐line treatment; patients without such an event were censored at either the start of second‐line treatment or at the last documented contact. OS was defined as the interval between start of first‐line treatment until death from any cause. Data of patients alive or lost to follow‐up were censored at the last documented contact. The median observation time was calculated using the reverse Kaplan‐Meier estimate. 32 Confidence limits for the survivor function were calculated employing a log‐log transformation. 33 Confidence intervals for median survival were calculated as described by Brookmeyer and Crowley. 34 All analyses were performed using Dell, Inc. (2016), Dell Statistica, version 13.1. software.dell.com and SAS software, version 9.4 of the SAS System for Windows (Copyright© 2002‐2012 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, North Carolina).
3. RESULTS
3.1. Patient demographics and clinical characteristics
Table 1 presents the demographics and clinical characteristics of patients with WM included into this analysis (n = 139). Most patients were male (62%); the median age at start of first‐line treatment was 72 years. In total, 66% of patients experienced at least one concomitant disease at start of first‐line treatment, mainly arterial hypertension (31%). A total of 28% of patients had comorbidities considered for the Charlson comorbidity index (CCI; CCI ≥1). In total, 28% of patients were in good general condition (ECOG = 0), whereas for 10% a poor performance status (ECOG ≥2) was reported. B‐symptoms were present in almost one‐third of patients (30%).
TABLE 1.
Patient demographics and clinical characteristics at start of first‐line treatment
| Characteristic | Patients with WM (n = 139) | ||
|---|---|---|---|
| Age | Years | Min‐Max | |
| Median | 71.5 | 37.1 to 93.4 | |
| n | % | ||
| <65 y | 41 | 29.5 | |
| 65 to 74 y | 49 | 35.3 | |
| ≥75 y | 49 | 35.3 | |
| kg/m 2 | SD | ||
| BMI, mean a | 24.9 | 4.0 | |
| n | % | ||
| Missing | 2 | 1.4 | |
| Sex | |||
| Female | 53 | 38.1 | |
| Male | 86 | 61.9 | |
| Patients with comorbidity | |||
| Any comorbidity b | 92 | 66.2 | |
| CCI = 0 c | 99 | 71.2 | |
| CCI ≥1 c | 39 | 28.1 | |
| Hypertension | 43 | 30.9 | |
| Cardiac disorders d | 19 | 13.7 | |
| Diabetes | 13 | 9.4 | |
| Chronic pulmonary disease | 11 | 7.9 | |
| Performance status | |||
| ECOG = 0 | 39 | 28.1 | |
| ECOG = 1 | 78 | 56.1 | |
| ECOG ≥2 | 14 | 10.1 | |
| Unknown | 8 | 5.8 | |
| B symptoms e | |||
| Present | 41 | 29.5 | |
| Unknown | 4 | 2.9 | |
| Haemoglobin | |||
| <12 g/dL | 96 | 69.1 | |
| LDH | |||
| >ULN | 35 | 25.2 | |
| Unknown | 5 | 3.6 | |
| Median time from diagnosis to treatment | Months | 25%/ 75% quantiles | |
| 1.6 | 0.7‐22.3 | ||
Note: Some percentages might not add up to 100% due to rounding.
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; SD, standard deviation; ULN, upper limit of normal; WM, Waldenström's macroglobulinaemia.
At enrolment.
At least one comorbidity according to Charlson and/or additional concomitant diseases.
Charlson comorbidity index (CCI) according to Quan et al35, 36; WM (two points) was not counted as a comorbidity.
Heart insufficiency, myocardial infarction, coronary artery disease and other cardiac disorders.
Fever, night sweats, loss of weight.
3.2. Choice of systemic treatment
3.2.1. First‐line treatment
Figure 2 displays the most frequently used first‐line treatments between 2009 and 2014 (n = 139), clustered by substance groups.
FIGURE 2.
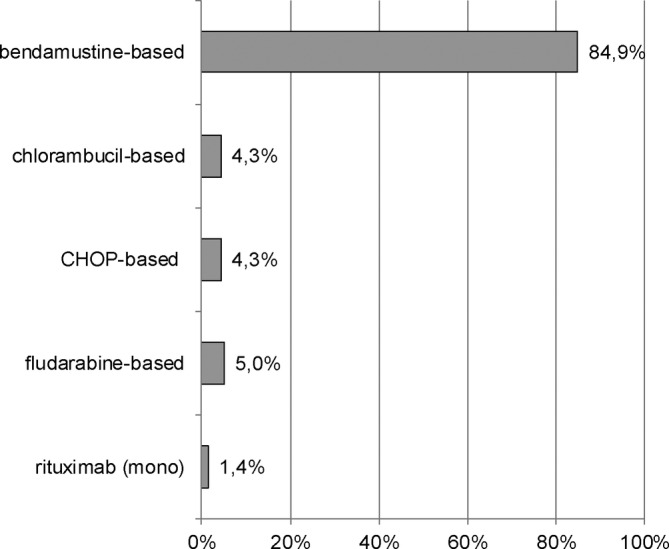
Choice of systemic first‐line treatment in Waldenström's macroglobulinaemia. Shown are first‐line treatments/treatment combinations between 2009 and 2014 sorted by relative frequency (n = 139). Bendamustine‐based: bendamustine (mono), bendamustine + rituximab (±prednisone/dexamethasone); chlorambucil‐based: chlorambucil (mono), chlorambucil + rituximab (±prednisone/mitoxantrone); fludarabine‐based: fludarabine (mono), fludarabine + cyclophosphamide (±rituximab); CHOP‐based: variations of CHOP (CHOP, CH, COP, HOP) ± rituximab (±prednisone/dexamethasone). Percentages may not add up to 100% due to rounding. C, cyclophosphamide; H, doxorubicin; O, vincristine; P, prednisone
By far, bendamustine‐based therapies were the most common first‐line treatments (85%, n = 118), with bendamustine combined with rituximab used for most of patients (81%, n = 112). Of those patients who received rituximab‐bendamustine, bendamustine was used for a median of six cycles (interquartile range [IQR] 1.0), rituximab for a median of six cycles (IQR 2.0). Our data show a trend towards an increasing use of rituximab‐bendamustine in course of the observation period (data on file). However, more than 60% of patients were treated with this combination already in 2009. Overall, other treatments were assigned to 15% of patients (n = 21). These treatments were based on chlorambucil (4%, n = 6) or cyclophosphamide/doxorubicin/vincristine/prednisone (CHOP; 4%, n = 6) or fludarabine (5%, n = 7), all of them mainly combined with rituximab. Rituximab monotherapy was used in two patients (1%). Due to rounding, individual percentages presented here may not add up to 100%.
3.2.2. Second‐line treatment
Second‐line treatment was documented for 19% of patients (n = 26) at the time of analysis, while 13% (n = 18) had died prior to receiving a second‐line treatment. The remainder were either still in first‐line treatment (potentially receiving more lines of treatment) or had been lost to follow‐up after first‐line treatment. From 2010 to 2017, patients most frequently received therapies based on bendamustine (31%, n = 8) as second‐line treatment, particularly rituximab‐bendamustine (23%, n = 6). Seven patients (27%) were treated with a regimen based on CHOP (mainly plus rituximab, R‐CHOP), five patients (19%) received fludarabine monotherapy or in combination with other substances. Ibrutinib, approved in October 2014, was prescribed to three patients (12%). Since a total of five patients had been recruited after the approval date of ibrutinib, this corresponds to a prescription rate of 60%. One patient (4%) received chlorambucil, two patients (8%) another treatment option: dexamethasone/high‐dose cytarabine (Ara‐C)/cisplatin (DHAP) combined with rituximab or a cyclophosphamide‐/etoposide‐based chemotherapy with rituximab.
3.3. Sequential treatment
The three most frequently used sequences for patients proceeding from first‐line to second‐line treatment were: bendamustine‐based therapy followed by (a) a bendamustine‐based treatment (23%, n = 6), or (b) a CHOP‐based treatment (23%, n = 6), or (c) a fludarabine‐based treatment (15%, n = 4).
3.4. Best response, PFS and OS
Outcome data are displayed in Table 2. The objective response rate for patients encompassing any positive response was 76%, with unconfirmed complete response (CRu) of 17%. For 13 patients (9%), stable disease has been reported, whereas for 6 patients (4%), progressive disease has been documented as the best response achieved during first‐line treatment.
TABLE 2.
OS, PFS and best response since start of first‐line treatment
| Characteristic | Patients with WM (n = 139) | |
|---|---|---|
| Best response | n | % |
| CRu a | 24 | 17.3 |
| PR | 82 | 59.0 |
| SD | 13 | 9.4 |
| PD | 6 | 4.3 |
| Unknown | 14 | 10.1 |
| Progression‐free survival | n | % |
| Events | 37 | 26.6 |
| Median PFS b | Months | 95% CI |
| NA | NA | |
| Survival rate | % | 95% CI |
| 12 months | 91.5 | 85.2 to 95.2 |
| 24 months | 86.5 | 79.1 to 91.4 |
| 36 months | 82.5 | 74.4 to 88.3 |
| Overall survival | n | % |
| Events | 25 | 18.0 |
| Median OS | Months | 95% CI |
| NA | NA | |
| Survival rate | % | 95% CI |
| 12 months | 95.5 | 90.3 to 98.0 |
| 24 months | 90.0 | 83.3 to 94.0 |
| 36 months | 87.1 | 79.8 to 91.9 |
| Median duration of observation | Months | 95% CI |
| 60.3 | 59.5 to 61.1 | |
Note: Some percentages might not add up to 100% due to rounding.
Abbreviations: CI, confidence interval; CRu, unconfirmed complete response; NA, not applicable; OS, overall survival; PD, progression; PFS, progression‐free survival; PR, partial response; SD, stable disease; WM, Waldenström's macroglobulinaemia.
Assessment by study sites, no evaluation by the criteria used in clinical trials.
Assessment by study sites and not according to response criteria used in clinical trials.
Median duration of observation was 60.3 months (95% confidence interval [CI] 59.5‐61.1). For both OS and PFS, the median was not reached. Three‐year PFS of patients was 83% (95% CI 74%‐88%; Table 2 and Figure 3), 3‐year OS was 87% (95% CI 80%‐92%; Table 2 and Figure 4).
FIGURE 3.
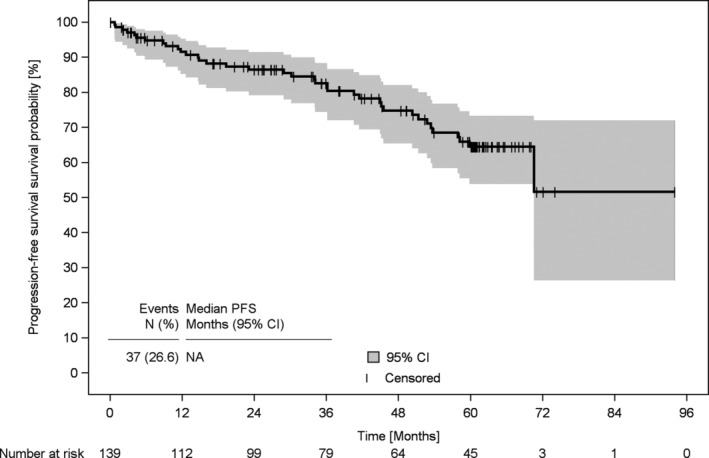
Progression‐free survival of patients with Waldenström's macroglobulinaemia since start of first‐line treatment. Progression‐free survival of patients with WM who had been enrolled at the beginning of their first‐line treatment (n = 139). CI, confidence interval; NA, not applicable; PFS, progression‐free survival, WM, Waldenström's macroglobulinaemia
FIGURE 4.
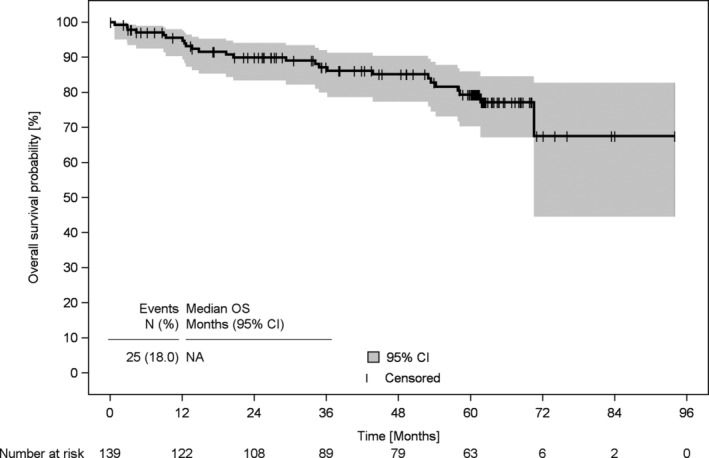
Overall survival of patients with Waldenström's macroglobulinaemia since start of first‐line treatment. Overall survival of patients with WM who had been enrolled at the beginning of their first‐line treatment (n = 139). CI, confidence interval; NA, not applicable; OS, overall survival; WM, Waldenström's macroglobulinaemia
At data cut‐off, 25 patients (18%) had died, 18 (13%) were still being observed, 36 (26%) were lost to follow‐up and 60 (43%) were alive at the end of the individual 5‐year observation period.
4. DISCUSSION
Waldenström's macroglobulinaemia is a rare haematological disorder for which only little prospective phase III evidence on its management exists and thus a clear standard of care for first‐line treatment is lacking. We present prospective real world data on treatment and survival of patients with WM outside a clinical trial setting in Germany. We show that rituximab‐bendamustine was by far the most frequently used first‐line treatment (81%). Second‐line treatment was more heterogenous and was mainly based on bendamustine, CHOP, fludarabine or ibrutinib which was approved 5 years after the start of this study. Three‐year PFS and 3‐year OS were 83% (95% CI 74%‐88%) and 87% (95% CI 80%‐92%), respectively.
Strengths of this study are the prospective, longitudinal design and the non‐selected participation of haematologists/oncologists across Germany recruiting into a large study cohort. This allows the analysis of smaller subsets of patients, such as the WM patient population. Nonetheless, there are limitations. In the TLN, only patients with WM who received systemic treatment had been included and not those with a watch‐and‐wait approach and no systemic treatment at all. Therefore, characteristics of our cohort are not representative of the whole WM patient population. Since data on the time of diagnosis were documented retrospectively, the median time between diagnosis and start of first‐line treatment may be underestimated, because patients with a long period of time until start of treatment may be underrepresented in the TLN: not all participating centres were possibly aware of the inclusion of such patients as soon as the need for treatment arose and therefore did not consider them for inclusion into the registry in the same way as patients for whom systemic treatment started close to diagnosis. Some prognostic factors for calculating the ISSWM, such as beta‐2 microglobulin and platelet count, had not been documented within the TLN. Since WM is a rare disorder, the number of patients included into this analysis is rather small compared to more common types of lymphomas and percentages provided in the results section should be interpreted with some caution. There were no specifications as to the timing, frequency or criteria of tumour assessment. Thus, clinical PFS data could be considered as the best clinical approximation, but might not be identical to the PFS determined in prospectively RCTs. The same holds true for data on CRu.
Our data show that the vast majority of patients with WM in German routine practice received first‐line treatment with rituximab‐bendamustine, with six cycles in median. This is in accordance with former and current guidelines recommending four to six cycles of rituximab combined with bendamustine as one primary first‐line treatment option for ‘medically fit’ patients (in addition to plasmapheresis in case of hyperviscosity).1, 37, 38, 39 In a randomised phase III study, patients with rituximab‐bendamustine in first‐line had longer PFS and OS than those who underwent therapy with R‐CHOP. 14 Since rituximab‐bendamustine has been associated with a prolonged PFS and time‐to‐next therapy, deep responses (complete response + very good partial response rates), and favourable toxicity profile, this combination is currently the primary regimen of choice for previously untreated WM.39, 40
Our findings on treatment patterns vary from those of the Swedish Lymphoma Registry (SLR) study on prognostic factors and treatment for WM that—to our knowledge—is the only prospective population‐based study published so far. 11 Of 203 patients with documented systemic first‐line treatment between 2000 and 2014, 153 were treated between 2007 and 2014 and mainly received cyclophosphamide‐(rituximab)‐based regimens (28%, of which 6% were R‐CHOP‐like regimens) and chlorambucil (27%, mostly without rituximab). Only in 7% of patients rituximab‐bendamustine was used. 11 These differences might reflect differences in patient cohorts, national guidelines and health care systems between Sweden and Germany.
Among the existing retrospective, mainly population‐based studies on treatment and outcome patterns of patients with WM,9, 24, 26, 27, 40, 41, 42, 43, 44 one large chart‐based study also included patients from German clinical practice. 9 In this study, R‐CHOP and rituximab‐bendamustine were the most frequently used chemoimmunotherapy regimens in first‐line which is similar to our findings. Of the 454 patients who had been treated in European academic and community centres from 2000 to 2014, 66 patients were treated in Germany (38%, n = 25 in community centres). 9
In our study, second‐line treatment was mostly based on bendamustine (primarily in combination with rituximab), CHOP or fludarabine. This is in line with German guidelines valid during the observation period 37 and similar to the results observed in the afore‐mentioned chart‐based study, with rituximab‐bendamustine and cyclophosphamide‐/CHOP‐based regimens used most frequently for second‐line chemoimmunotherapy, followed by regimens based on fludarabine. 9 As for ibrutinib which was used for second‐line treatment in more than half of patients who had been recruited after the approval date of ibrutinib in October 2014, our data clearly show that newly licensed agents are quickly implemented into routine practice. This is even more noteworthy, as the uptake of ibrutinib for relapsed WM disease into consensus publications and guidelines took some time.
The PFS data presented here (2‐year PFS of 87%; 3‐year PFS of 83%) are in line with recent retrospective data in patients with WM after first‐line treatment with rituximab‐bendamustine revealing a 2‐year PFS of 88% 40 and a 3‐year PFS of approximately 85%. 27 In the phase III RCT on bendamustine plus rituximab vs R‐CHOP, those patients with WM who received bendamustine plus rituximab in first‐line (n = 22) had a 3‐year PFS of about 80%. 14 The latter finding is even more surprising, since patients treated in routine practice usually markedly differ from those selected for clinical trials30, 45 and results suggest that patient outcomes are worse outside a clinical trial setting. 9 Interestingly, in the above‐mentioned retrospective chart review, PFS was shortened in patients treated in academic centres compared to those treated in community institutions, but this result might have been confounded by factors to be explored. 9
Three‐year OS of patients included in our analysis was 87%, a finding that agrees with results from other (retrospective and prospective) studies: in patients with WM who received any systemic first‐line treatment within the period of 2000 to 2014, 3‐year OS ranged from 63% to approximately 95%.9, 11, 26, 27, 41, 43 Notably, the lowest 3‐year OS has been observed in patients with the ‘highest’ median age of 78 years 43 and the highest 3‐year OS in patients of the ‘youngest’ median age (65 years). 9 With 72 years in median at start of first‐line treatment, the age of our registry cohort is very similar to that of the prospective SLR cohort having a median age of 73 years. 11 Even though first‐line treatments differ between this study and the SLR study, both the median observation time and the 3‐year OS are highly comparable: 60.3 months vs 55.5 months 11 and 87% vs approximately 80%. 11 Since patients' age has been found to be one of the strongest prognostic factors, 11 our data also suggest an important role of age for WM outcome.
5. CONCLUSION
Since there is only little evidence from prospective phase III RCTs, the prospective data we present here provide important insights into treatment and survival of patients with WM outside a clinical trial setting in Germany. We show that bendamustine plus rituximab, with a median of six cycles, was by far the most commonly used first‐line treatment corresponding to former and current guideline recommendations. Our findings indicate that a newly licensed agent like ibrutinib is quickly implemented into routine practice. Outcome data of this analysis agree with recent retrospective and prospective (observational and clinical trial) data. When patient's participation in clinical trials is not possible, real world data can help bridging the gap of evidence, especially in rare diseases.
CONFLICT OF INTEREST
Hans Rainer Slawik, Ute Bückner, Annette Sauer, Mark‐Oliver Zahn, Natalie Wetzel, Anja Kaiser‐Osterhues and Leonora Houet declare no conflict of interest concerning the topic of this publication. Wolfgang Knauf has received honoraria by Mundipharma and Janssen for talks and attendance of conferences. Wolfgang Abenhardt has received financial support from Roche, Amgen, Celgene and Gilead Sciences in the context of a lymphoma symposium. Burkhard Otremba declares stock ownership of iOMEDICO. Norbert Marschner is Chief Executive Officer of iOMEDICO and holds shares of this company.
AUTHOR CONTRIBUTIONS
Wolfgang Knauf, Wolfgang Abenhardt, Leonora Houet, Natalie Wetzel, Anja Kaiser‐Osterhues and Norbert Marschner designed the research study. Wolfgang Knauf, Wolfgang Abenhardt, Hans Rainer Slawik, Ute Bückner, Burkhard Otremba, Annette Sauer, Mark‐Oliver Zahn and Norbert Marschner performed the research. Natalie Wetzel, Leonora Houet and Anja Kaiser‐Osterhues analysed the data. Anja Kaiser‐Osterhues wrote the paper. All the authors critically reviewed and approved the manuscript.
ETHICS STATEMENT
All experiments comply with the current laws in Germany, where they were performed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments. This study was approved by the responsible ethics committee and is registered at ClinicalTrials.gov (NCT00889798).
ORIGINAL PUBLICATION
The authors confirm that this work has not been published previously. Parts of it have been presented at the German annual meeting of the DGHO German Society for Hematology and Medical Oncology eV, 2018 (poster presentation).
ACKNOWLEDGEMENTS
The authors would like to thank all patients, physicians and study teams participating in the TLN. We thank Renate Grugel (iOMEDICO) for support during project management, data analysis and critical review of the manuscript and Dr Martina Jänicke (iOMEDICO) for critical comments on the design of the research study and review of the manuscript. The TLN is designed, managed and analysed by iOMEDICO and has received continuous financial support from Celgene GmbH, Mundipharma GmbH, Onkovis GmbH and Roche Pharma AG. None of the funding companies had any role in study design, data collection and analysis, interpretation of results, decision to publish, or preparation of the manuscript. The TLN‐Group collaborates with the Arbeitskreis Klinische Studien in onkologischen und hämatologischen Praxen e.V. (AKS) and the Kompetenznetz Maligne Lymphome (KML).
Knauf W, Abenhardt W, Slawik HR, et al. Rare lymphomas in routine practice—Treatment and outcome in Waldenström's macroglobulinaemia in the prospective German Tumour Registry Lymphatic Neoplasms. Hematological Oncology. 2020;38:344–352. 10.1002/hon.2740
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1002/hon.2740.
Funding information Celgene GmbH; Mundipharma GmbH; Onkovis GmbH; Roche Pharma AG
REFERENCES
- 1. Kastritis E, Leblond V, Dimopoulos MA, et al. Waldenström's macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2018;29(Supplement_4):iv41‐iv50. 10.1093/annonc/mdy146. [DOI] [PubMed] [Google Scholar]
- 2. Kapoor P, Paludo J, Vallumsetla N, Greipp PR. Waldenström macroglobulinemia: what a hematologist needs to know. Blood Rev. 2015;29(5):301‐319. 10.1016/j.blre.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375‐2390. 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spinner MA, Varma G, Advani RH. Novel approaches in Waldenström Macroglobulinemia. Hematol Oncol Clin North Am. 2018;32(5):875‐890. 10.1016/j.hoc.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 5. Hunter ZR, Xu L, Yang G, et al. The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM‐like CXCR4 mutations, and small somatic deletions associated with B‐cell lymphomagenesis. Blood. 2014;123(11):1637‐1646. 10.1182/blood-2013-09-525808. [DOI] [PubMed] [Google Scholar]
- 6. Phekoo KJ, Jack RH, Davies E, Møller H, Schey SA, South Thames Haematology Specialist Committee . The incidence and survival of Waldenström's Macroglobulinaemia in South East England. Leuk Res. 2008;32(1):55‐59. 10.1016/j.leukres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 7. Robert Koch‐Institut. Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V., eds. Krebs in Deutschland 2009/2010. Häufigkeiten und Trends. 9. Ausgabe. Berlin: Robert Koch‐Institut; 2013. [Google Scholar]
- 8. Gemeinsamer Bundesausschuss (G‐BA) . Dossier zur Nutzenbewertung gemäß § 35a SGB V, Ibrutinib (IMBRUVICA®), Janssen‐Cilag GmbH, Modul 3C. Berlin; 2016. https://www.g‐ba.de/downloads/92‐975‐1338/2016‐01‐29_Modul3C_Ibrutinib.pdf. Accessed November 13, 2019.
- 9. Buske C, Sadullah S, Kastritis E, et al. Treatment and outcome patterns in European patients with Waldenström's macroglobulinaemia: a large, observational, retrospective chart review. Lancet Haematol. 2018;5(7):e299‐e309. 10.1016/S2352-3026(18)30087-5. [DOI] [PubMed] [Google Scholar]
- 10. Dimopoulos MA, Tedeschi A, Trotman J, et al. Phase 3 trial of Ibrutinib plus rituximab in Waldenström's Macroglobulinemia. N Engl J Med. 2018;378(25):2399‐2410. 10.1056/NEJMoa1802917. [DOI] [PubMed] [Google Scholar]
- 11. Brandefors L, Melin B, Lindh J, Lundqvist K, Kimby E. Prognostic factors and primary treatment for Waldenström macroglobulinemia—a Swedish Lymphoma Registry study. Br J Haematol. 2018;183(4):564‐577. 10.1111/bjh.15558. [DOI] [PubMed] [Google Scholar]
- 12. Leblond V, Johnson S, Chevret S, et al. Results of a randomized trial of chlorambucil versus fludarabine for patients with untreated Waldenström macroglobulinemia, marginal zone lymphoma, or lymphoplasmacytic lymphoma. J Clin Oncol. 2013;31(3):301‐307. 10.1200/JCO.2012.44.7920. [DOI] [PubMed] [Google Scholar]
- 13. Buske C, Hoster E, Dreyling M, et al. The addition of rituximab to front‐line therapy with CHOP (R‐CHOP) results in a higher response rate and longer time to treatment failure in patients with lymphoplasmacytic lymphoma: results of a randomized trial of the German Low‐Grade Lymphoma Study Group (GLSG). Leukemia. 2009;23(1):153‐161. 10.1038/leu.2008.261. [DOI] [PubMed] [Google Scholar]
- 14. Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first‐line treatment for patients with indolent and mantle‐cell lymphomas: an open‐label, multicentre, randomised, phase 3 non‐inferiority trial. Lancet. 2013;381(9873):1203‐1210. 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 15. Kyle RA, Greipp PR, Gertz MA, et al. Waldenström's macroglobulinaemia: a prospective study comparing daily with intermittent oral chlorambucil. Br J Haematol. 2000;108(4):737‐742. 10.1046/j.1365-2141.2000.01918.x. [DOI] [PubMed] [Google Scholar]
- 16. Treon SP, Castillo JJ. The real world of Waldenström's macroglobulinaemia. Lancet Haematol. 2018;5(7):e275‐e276. 10.1016/S2352-3026(18)30091-7. [DOI] [PubMed] [Google Scholar]
- 17. Dimopoulos MA, Kastritis E, Owen RG, et al. Treatment recommendations for patients with Waldenström macroglobulinemia (WM) and related disorders: IWWM‐7 consensus. Blood. 2014;124(9):1404‐1411. 10.1182/blood-2014-03-565135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Owen RG, Pratt G, Auer RL, et al. Guidelines on the diagnosis and management of Waldenström macroglobulinaemia. Br J Haematol. 2014;165(3):316‐333. 10.1111/bjh.12760. [DOI] [PubMed] [Google Scholar]
- 19. Dimopoulos MA, Anagnostopoulos A, Kyrtsonis M‐C, et al. Primary treatment of Waldenström macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol. 2007;25(22):3344‐3349. 10.1200/JCO.2007.10.9926. [DOI] [PubMed] [Google Scholar]
- 20. Dimopoulos MA, García‐Sanz R, Gavriatopoulou M, et al. Primary therapy of Waldenstrom macroglobulinemia (WM) with weekly bortezomib, low‐dose dexamethasone, and rituximab (BDR): long‐term results of a phase 2 study of the European Myeloma Network (EMN). Blood. 2013;122(19):3276‐3282. 10.1182/blood-2013-05-503862. [DOI] [PubMed] [Google Scholar]
- 21. Treon SP, Gustine J, Meid K, et al. Ibrutinib monotherapy in symptomatic, treatment‐Naïve patients with Waldenström Macroglobulinemia. J Clin Oncol. 2018;36(27):2755‐2761. 10.1200/JCO.2018.78.6426. [DOI] [PubMed] [Google Scholar]
- 22. Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenström's macroglobulinemia. N Engl J Med. 2015;372(15):1430‐1440. 10.1056/NEJMoa1501548. [DOI] [PubMed] [Google Scholar]
- 23. Morel P, Duhamel A, Gobbi P, et al. International prognostic scoring system for Waldenstrom macroglobulinemia. Blood. 2009;113(18):4163‐4170. 10.1182/blood-2008-08-174961. [DOI] [PubMed] [Google Scholar]
- 24. Kastritis E, Kyrtsonis M‐C, Morel P, et al. Competing risk survival analysis in patients with symptomatic Waldenström macroglobulinemia: the impact of disease unrelated mortality and of rituximab‐based primary therapy. Haematologica. 2015;100(11):e446‐e449. 10.3324/haematol.2015.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kastritis E, Morel P, Duhamel A, et al. A revised international prognostic score system for Waldenström's macroglobulinemia. Leukemia. 2019;33:2654‐2661. 10.1038/s41375-019-0431-y. [DOI] [PubMed] [Google Scholar]
- 26. Castillo JJ, Olszewski AJ, Kanan S, Meid K, Hunter ZR, Treon SP. Overall survival and competing risks of death in patients with Waldenström macroglobulinaemia: an analysis of the Surveillance, Epidemiology and End Results database. Br J Haematol. 2015;169(1):81‐89. 10.1111/bjh.13264. [DOI] [PubMed] [Google Scholar]
- 27. Castillo JJ, Gustine JN, Meid K, et al. Response and survival for primary therapy combination regimens and maintenance rituximab in Waldenström macroglobulinaemia. Br J Haematol. 2018;181(1):77‐85. 10.1111/bjh.15148. [DOI] [PubMed] [Google Scholar]
- 28. Knauf W, Abenhardt W, Aldaoud A, et al. Treatment of non‐transplant patients with multiple myeloma: routine treatment by office‐based haematologists in Germany—data from the prospective Tumour Registry Lymphatic Neoplasms (TLN). Oncol Res Treat. 2014;37(11):635‐644. 10.1159/000368315. [DOI] [PubMed] [Google Scholar]
- 29. Knauf W, Abenhardt W, Dörfel S, et al. Routine treatment of patients with chronic lymphocytic leukaemia by office‐based haematologists in Germany‐data from the Prospective Tumour Registry Lymphatic Neoplasms. Hematol Oncol. 2015;33(1):15‐22. 10.1002/hon.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knauf W, Aldaoud A, Hutzschenreuter U, et al. Survival of non‐transplant patients with multiple myeloma in routine care differs from that in clinical trials‐data from the prospective German Tumour Registry Lymphatic Neoplasms. Ann Hematol. 2018;97(12):2437‐2445. 10.1007/s00277-018-3449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Knauf W, Abenhardt W, Mohm J, et al. Similar effectiveness of R‐CHOP‐14 and ‐21 in diffuse large B‐cell lymphoma—data from the prospective German Tumour Registry Lymphatic Neoplasms. Eur J Haematol. 2019;103(5):460‐471. 10.1111/ejh.13295. [DOI] [PubMed] [Google Scholar]
- 32. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials. 1996;17(4):343‐346. 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 33. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley and Sons; 1980. [Google Scholar]
- 34. Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29‐41. 10.2307/2530286. [DOI] [Google Scholar]
- 35. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373‐383. 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 36. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676‐682. 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 37. Buske C, Herold M, Rummel M, Dreyling M. DGHO‐Leitlinie Morbus Waldenström. Berlin: Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie e.V. (DGHO); 2010. [Google Scholar]
- 38. Buske C, Heim M, Herold M, Staber PB, Dreyling M. DGHO‐Leitlinie Morbus Waldenström (Lymphoplasmozytisches Lymphom). Berlin: Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie e.V. (DGHO); 2018. [Google Scholar]
- 39. Kapoor P, Ansell SM, Fonseca R, et al. Diagnosis and management of Waldenström macroglobulinemia: Mayo Stratification of Macroglobulinemia and Risk‐Adapted Therapy (mSMART) Guidelines 2016. JAMA Oncol. 2017;3(9):1257‐1265. 10.1001/jamaoncol.2016.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paludo J, Abeykoon JP, Shreders A, et al. Bendamustine and rituximab (BR) versus dexamethasone, rituximab, and cyclophosphamide (DRC) in patients with Waldenström macroglobulinemia. Ann Hematol. 2018;97(8):1417‐1425. 10.1007/s00277-018-3311-z. [DOI] [PubMed] [Google Scholar]
- 41. Kastritis E, Kyrtsonis M‐C, Hatjiharissi E, et al. No significant improvement in the outcome of patients with Waldenström's macroglobulinemia treated over the last 25 years. Am J Hematol. 2011;86(6):479‐483. 10.1002/ajh.22027. [DOI] [PubMed] [Google Scholar]
- 42. Kristinsson SY, Eloranta S, Dickman PW, et al. Patterns of survival in lymphoplasmacytic lymphoma/Waldenström macroglobulinemia: a population‐based study of 1,555 patients diagnosed in Sweden from 1980 to 2005. Am J Hematol. 2013;88(1):60‐65. 10.1002/ajh.23351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Olszewski AJ, Treon SP, Castillo JJ. Evolution of management and outcomes in Waldenström macroglobulinemia: a population‐based analysis. Oncologist. 2016;21(11):1377‐1386. 10.1634/theoncologist.2016-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Olszewski AJ, Chen C, Gutman R, Treon SP, Castillo JJ. Comparative outcomes of immunochemotherapy regimens in Waldenström macroglobulinaemia. Br J Haematol. 2017;179(1):106‐115. 10.1111/bjh.14828. [DOI] [PubMed] [Google Scholar]
- 45. Marschner N, Staehler M, Müller L, et al. Survival of patients with advanced or metastatic renal cell carcinoma in routine practice differs from that in clinical trials‐analyses from the German Clinical RCC Registry. Clin Genitourin Cancer. 2017;15(2):e209‐e215. 10.1016/j.clgc.2016.08.022. [DOI] [PubMed] [Google Scholar]


