Figure 2.
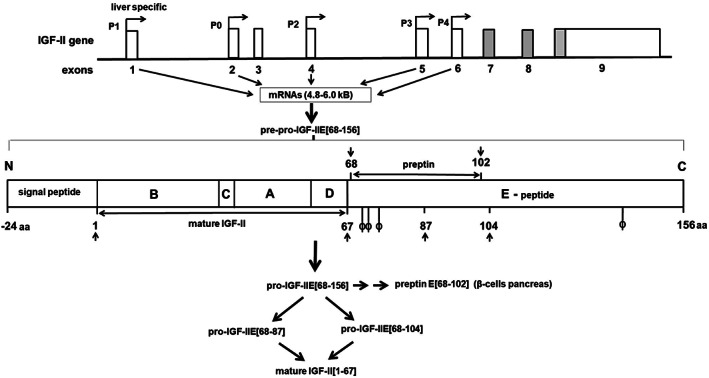
Schematic illustration of the structure of the insulin‐like growth factor (IGF)‐II gene, located on chromosome 11p15.5 with its five putative promotors, leading to mRNAs ranging between 4.8 and 6.0 kB. Only exons 7, 8, and a part of 9 encode (depicted in gray) for the pre‐pro‐IGF‐II translation product of 180 amino acids that is organized into five structural domains (A–E) and contains 3 intra‐molecular disulfide bonds between Ser residues 9 and 47, 21 and 60, and 46 and 51, respectively.5, 36 The A and B domains of mature IGF‐II are structurally analogous to mature IGF‐I and insulin. The flexible C domain is similar to the C domain in pro‐insulin but is not proteolytically cleaved, while the D domain is unique to IGF‐II. The N‐terminal signal peptide of 24 amino acids is enzymatically removed and the remaining pro‐IGF‐IIE [68–156] glycosylated, followed by sequential proteolysis (indicated by arrows) yielding mature IGF‐II1, 67 Demonstrated glycosylation sites are indicated by ϕ. Relatively stable, incompletely processed heterogeneously glycosylated or non‐glycosylated pro‐IGF‐II peptides that contain all or part of the E‐domain are also secreted, normally making up 10–20% of the total amount of circulating IGF‐II. In addition, in granules of pancreatic β cells the E‐domain derived peptide preptin [68–102] has been detected
