Scheme 1.
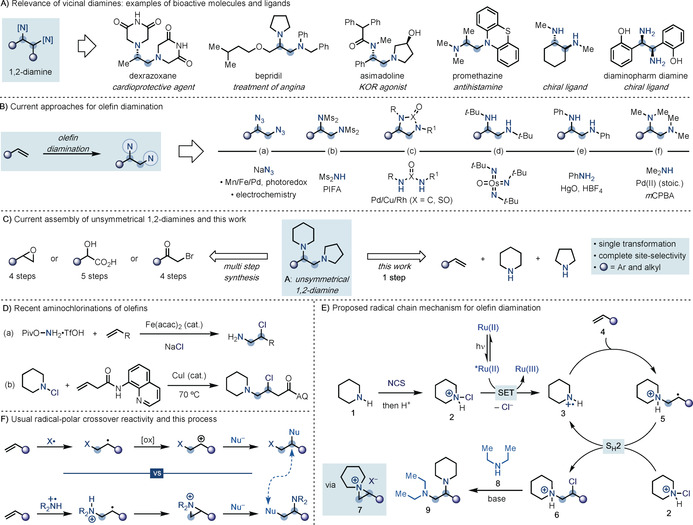
A) Vicinal diamines are high‐value materials in chemical sciences. B) Current strategies for olefin diamination do not enable the direct use of alkylamines. C) The construction of unsymmetrical diamines requires multistep syntheses while the strategy reported here assembles them in a single step. D) Recent developments for olefin aminochlorination. E) The proposed mechanism for olefin diamination involves the generation of an aminium radical to achieve an aminochlorination reaction, which is followed by aziridinium formation and regioselective ring‐opening. F) This radical‐polar crossover strategy offers a different disconnection to classical approaches.
