Scheme 5.
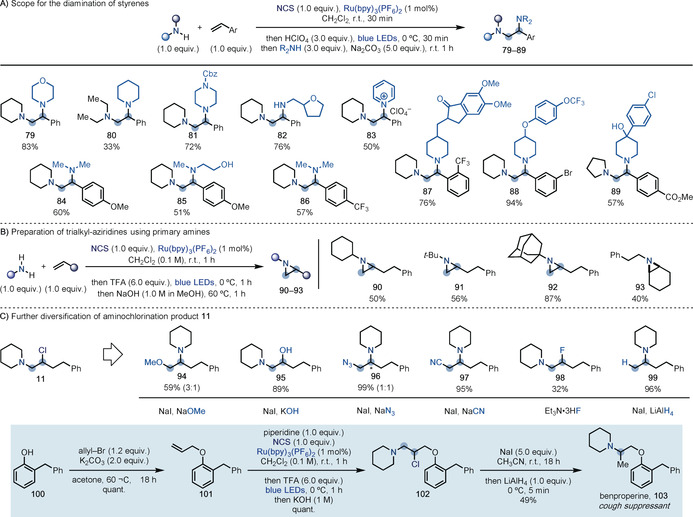
A) Styrene diamination provides a different regioselectivity. B) The use of primary amines enables the preparation of trialkyl aziridines. C) Further diversification of β‐chloroamine and use of the reduction process for the 3‐step synthesis of benproperine. [a] A basic work‐up was performed before addition of NaI and the amine. [b] Upon addition of NaI and the amine the mixture was warmed to 60 °C. [c] In this case the aziridinium was formed using Ag(BF4). * Denotes the minor constitutional isomer.
