Fig. 1.
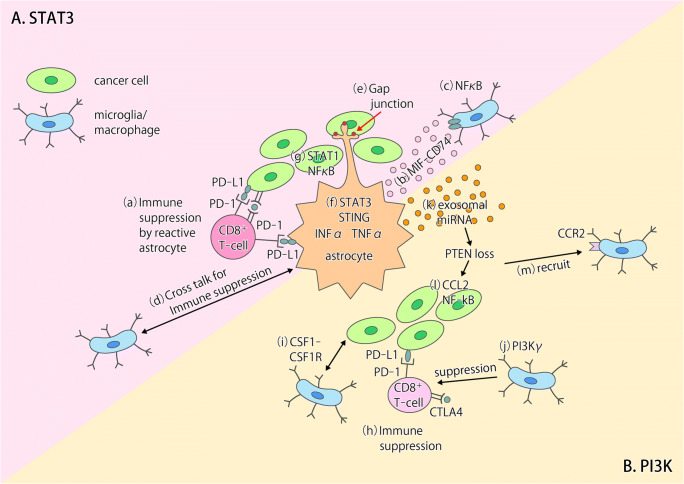
Models for the interactions between tumor cells and the tumor microenvironment in the brain. a The STAT3 and NF-κB signaling pathways play key roles in tumor-associated astrocytes in brain metastases. (a) Reactive astrocytes show phosphorylation of STAT3 and expression of PD-L1, which may contribute to the suppression of CD8+ T cell function [26]. (b) Reactive astrocytes positive for STAT3 activation increase the number of CD74+ microglia-macrophages in brain metastases through production of the CD74 ligand MIF and consequent activation of the MIF-CD74 axis [26]. (c) The NF-κB pathway is activated in CD74+ microglia-macrophages [26]. (d) Cross talk between microglia-macrophages and astrocytes contributes to establishment of an immunosuppressive environment in primary brain tumors [28]. (e) Cancer cells transfer cGAMP to astrocytes through Cx43-PCDH7 gap junctions, resulting in activation of the cGAS-STING pathway in the latter cells [32]. (f, g) Production of IFN-α and TNF-α by astrocytes induces activation of STAT1 and NF-κB pathways in cancer cells and thereby supports brain metastasis [32]. b. The PI3K-Akt signaling pathway plays a key role in brain metastases of breast cancer cells. (h) PI3K activation up-regulates PD-L1 and CTLA4 expression in cancer cells [33]. (i) Cross talk between cancer cells and macrophages results in activation of PI3K and CSF1-CSF1R signaling in macrophages [33]. (j) PI3Kγ signaling in macrophages inhibits NF-κB activation and promotes immune suppression in head and neck cancer [38]. (k) Loss of PTEN expression in cancer cells is induced epigenetically by exosomal miRNAs released from astrocytes [41]. (l, m) PTEN loss results in increased expression of the chemokine CCL2 and activation of NF-κB signaling in cancer cells as well as in the consequent CCR2-dependent recruitment of macrophages [41]