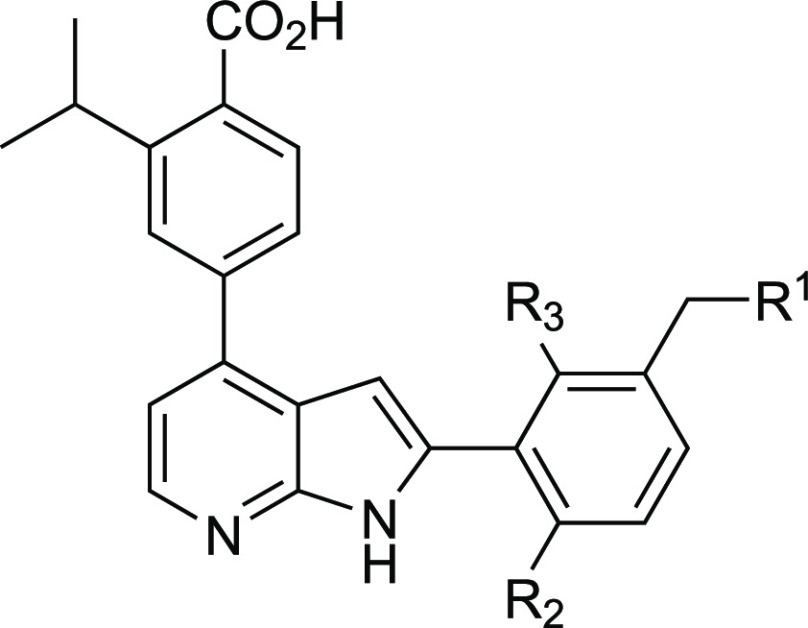
Table 1. Physicochemical Properties and Activity Data of TCMDC-135051 Ring A Analogues.
|
PfCLK3 |
3D7 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| analogue | R1 | R2 | R3 | IC50 (nM)a | pIC50 | EC50 (nM)b | pEC50 | log D7.4c | clint (mL/min/g liver)d |
| 1 | NEt2 | OMe | H | 40 | 7.4 (±0.221) | 180 | 6.7 (±0.126) | 0.93 | 1.12 |
| 8a | NMe2 | OMe | H | 29 | 7.5 (±0.224) | 457 | 6.3 (±0.129) | 0.85 | 2.53 |
| 8b | N-pyrrolidinyl | OMe | H | 38 | 7.4 (±0.113) | 382 | 6.4 (±0.081) | 2.43 | 1.94 |
| 8c | N-morpholinyl | OMe | H | 9 | 8.0 (±0.191) | 1339 | 5.9 (±0.118) | 1.20 | 1.60 |
| 12 | NH2 | OMe | H | 76 | 7.1 (±0.142) | 2801 | 5.6 (±0.104) | 0.61 | 2.92 |
| 15 | H | OMe | H | 79 | 7.1 (±0.132) | 1456 | 5.8 (±0.152) | 2.45 | 2.54 |
| 19 | NEt2 | OH | H | 22 | 7.7 (±0.115) | 3529 | 5.5 (±0.133) | 0.59 | 1.94 |
| 23 | NEt2 | H | H | 25 | 7.6 (±0.089) | 309 | 6.5 (±0.114) | 0.80 | 0.85 |
| 27 | NEt2 | H | OMe | 17 | 7.7 (±0.116) | 3167 | 5.6 (±0.109) | 0.74 | 1.65 |
IC50 (the concentration of an inhibitor where the response is reduced by half).
EC50 (the concentration of a drug that gives half-maximal response). IC50 and EC50 values are means ± SEM of three independent experiments run in triplicates (n = 3).
log D7.4 (distribution co-efficient) was estimated using HPLC chromatography.
In vitro intrinsic clearance in mouse liver microsomes.