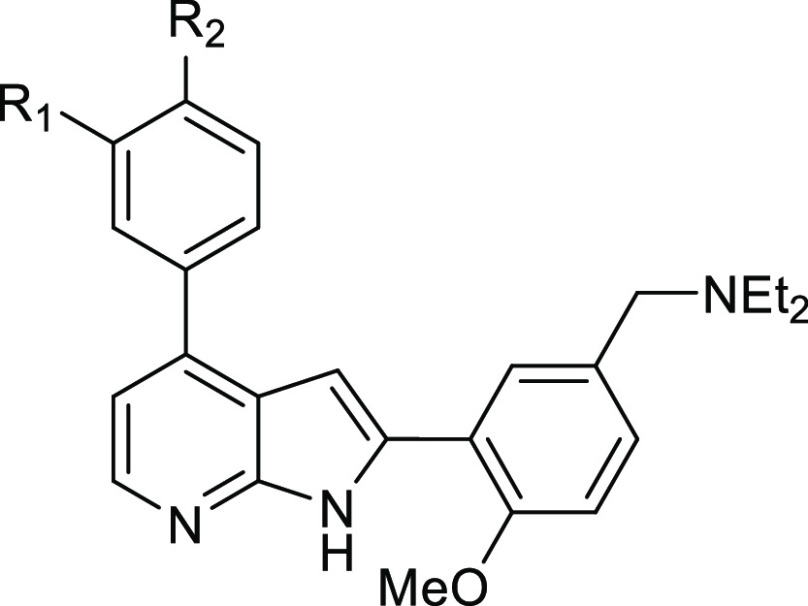
Table 2. Physicochemical Properties and Activity Data of TCMDC-135051 Ring B Analogues.
|
PfCLK3 |
3D7 |
|||||||
|---|---|---|---|---|---|---|---|---|
| analogue | R1 | R2 | IC50 (nM)a | pIC50 | ED50 (nM)b | pEC50 | log D7.4c | Clint (mL/min/g liver)d |
| 28 | CH3 | CO2H | 24 | 7.6 (±0.10) | 1185 | 5.6 (±0.097) | 0.66 | 1.33 |
| 29 | H | CO2H | 34 | 7.5 (±0.089) | 3272 | 5.5 (±0.124) | 0.65 | 11.01 |
| 30 | CH(CH3)2 | 1H-tetrazole | 19 | 7.7 (±0.089) | 270 | 6.6 (±0.158) | 0.93 | 2.32 |
| 31 | CH(CH3)2 | H | 1300 | 6.0 (±0.091) | NDe | NDe | 4.45 | 9.54 |
| 9 | CH(CH3)2 | CO2CH2CH3 | 390 | 6.4 (±0.087) | NDe | NDe | 2.45 | 16.06 |
| 32 | CO2H | CH(CH3)2 | 1385 | 4.9 (±0.093) | NDe | NDe | NDe | NDe |
IC50 (the concentration of an inhibitor where the response is reduced by half).
EC50 (the concentration of a drug that gives half-maximal response). IC50 and EC50 values are means ± SEM of three independent experiments run in triplicates (n = 3).
log D7.4 (distribution co-efficient) was estimated using HPLC chromatography.
In vitro intrinsic clearance in mouse liver microsomes.
ND, not determined.