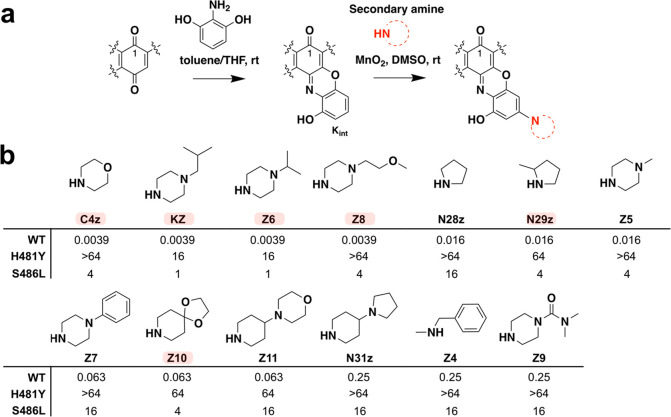
Figure 3.
Synthesis and activity of C-3/C-4 benzoxazino Kang derivatives. (a) Benzoxazino modification synthesis reaction. (b) Structural modifications and MIC values (μg/mL) of Kang benzoxazino derivatives against wild-type (WT) and RifRS. aureus strains. Amines used for synthesis are shown. The MIC values for the parent compound, Kang A, were 0.016 μg/mL, >64 μg/mL, and 0.25 μg/mL against the WT, H481Y, and S486L strains, respectively. A subset of the semisynthetic derivatives, highlighted in red, was subjected to downstream analyses. N29z was synthesized and screened as a diastereomeric mixture of 2-methylpyrrolidine.