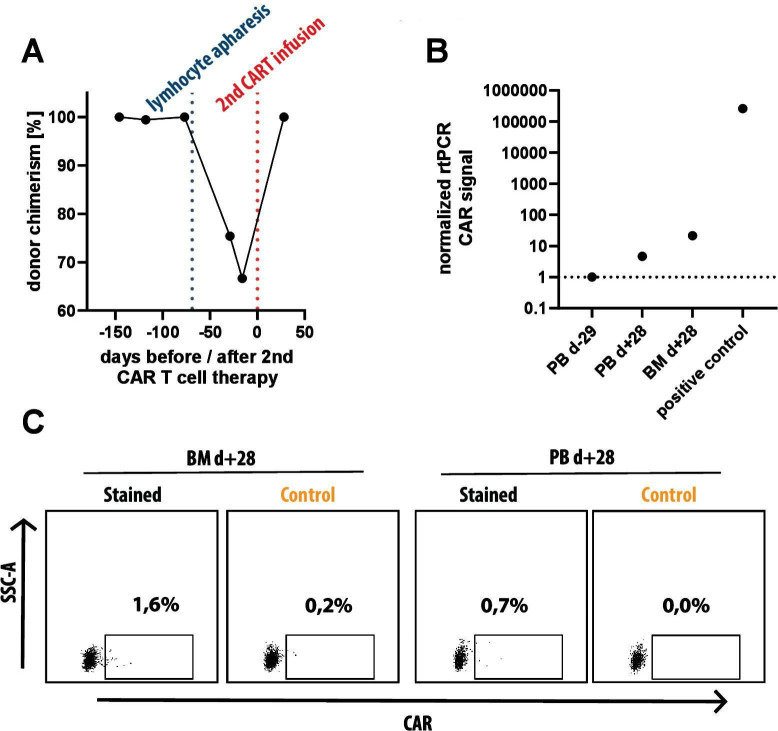
Figure 3.
Donor chimerism at time of lymphocyte apheresis and detection of CD19 targeted CAR T cells at time of ocular relapse. (A) Donor chimerism in peripheral blood as determined by Fluorescence in situ analysis analysis based on X/Y chromosome analysis with a female donor for a male patient. Shortly before lymphocyte apheresis from the patient (blue dotted line), 100% donor chimerism was documented. (B) Real-time PCR (RT-PCR) analysis of peripheral blood prior to second CAR T-cell therapy (PB d-29), peripheral blood (PB d+28) and bone marrow (BM d+28) 1 month after second CAR T-cell therapy and a positive control (in vitro generated CD19 CAR T-cells from our lab) using primers specific for the CAR molecule. A weak signal can be seen in both samples after second CAR T-cell therapy both in peripheral blood (4.6x negative control) and BM (21.5x negative control). (C) Flow cytometry analysis of the same two biosamples used for RT-PCR, taken at d +28 after second CAR T-cell therapy. after gating on lymphocytes (SSC-A vs FSC-A), single cells (FSC-H vs FSC-A), CD45hi, CD3+ and TCRalpha/beta+ we measured expression of CD19 CAR with recombinant, FITC-labeled CD19 molecule (Stained) and used recombinant, FITC-labeled CD22 molecule as negative control (Control). within BM, 1.4% of CAR T cells can be detected and 0.7% CAR T cells are detected within peripheral blood. CAR, chimeric antigen receptor, FSC-A, forward scatter area, FSC, forward scatter height.