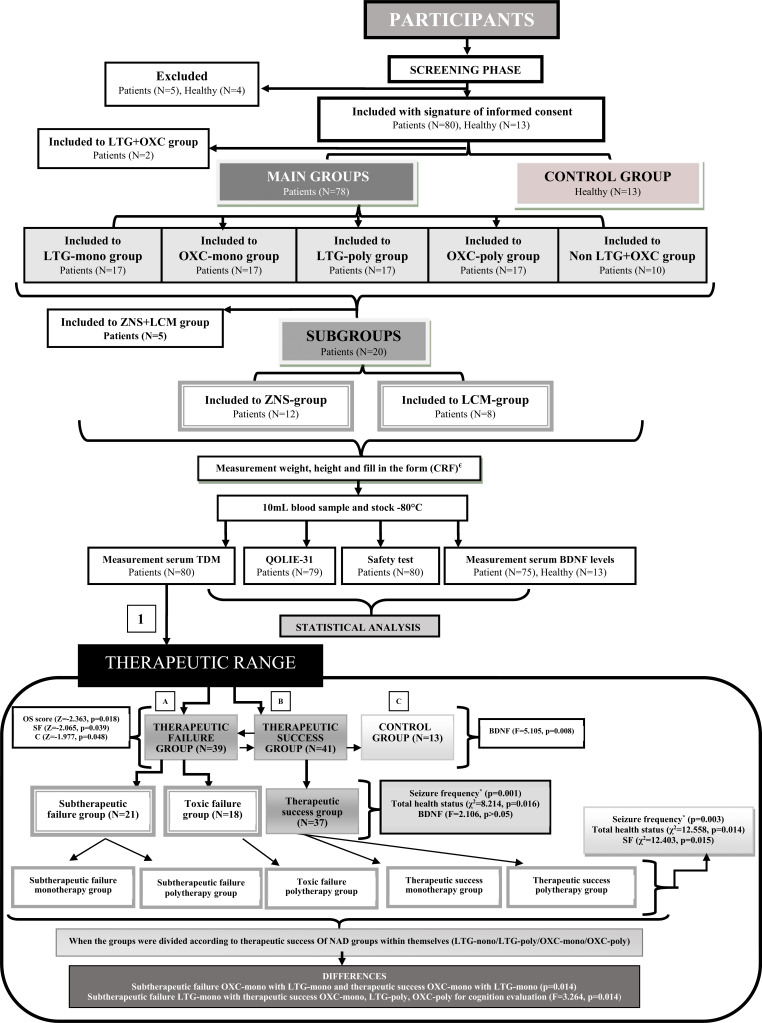
Fig. (1).
Study design with therapeutic success for AEDs. 1; All participants were divided into there groups: A) successfull in the therapeutic range, B) failure in the therapeutic range, C) control group. Therapeutic successfull group values were high. ±Total health status, OS, SF and C values were higher therapeutic success groups according to toxic failure groups. Control group was added to the each binary, triple and quinary groups for only BDNF comparisons. *Fisher exact test. €All participants. CRF; CASE Report Form, AED; Antiepileptic Drug NAD; New Antiepileptic Drug, OAD; Old Antiepileptic Drug, OD; Other Drug, Mono; Monotherapy, Poly; Polytherapy, BDNF; Brain-Derived Neurotrophic Factor, SW; Seizure Worry, OQOL; Overall Quality of Life, EWB; Emotional Well-Being, EF; Energy/Fatigue, C; Cognitive, ME; Medication Effects, SF; Social function, OS; Overall score, TDM; Therapeutic Drug Monitoring. AED; Antiepileptic Drug NAD; New Antiepileptic Drug, BDNF; brain-derived neurotropic factor, cognitive (C), social function (SF), overall score (OS).