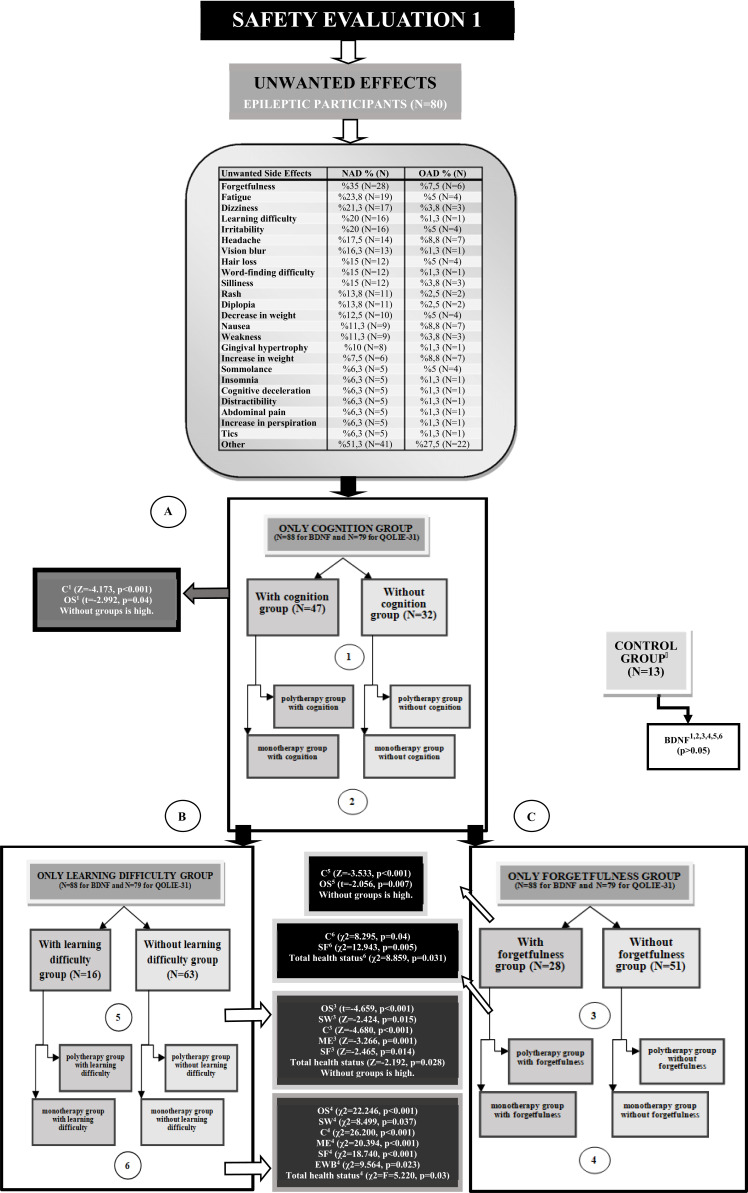
Fig. (2).
Safety evaluation and comparison for unwanted effects. A; All participants were divided into there groups: A1) cognition unwanted effect owner, A2) cognition unwanted effect not owner, A3) control group. B; All participants were divided into there groups: B1) forgetfulness unwanted effect owner, B2) forgetfulness unwanted effect not owner, B3) control group. C; All participants were divided into there groups: C1) learning difficulty unwanted effect owner, C2) learning difficulty unwanted effect not owner, C3) control group. Control group was added to the each binary and quaternary groups for only BDNF comparisons and user ANOVA test. AED; Antiepileptic Drug NAD; New Antiepileptic Drug, OAD; Old Antiepileptic Drug, OD; Other Drug, Mono; Monotherapy, Poly; Polytherapy, BDNF; Brain-Derived Neurotrophic Factor, SW; Seizure Worry, OQOL; Overall Quality of Life, EWB; Emotional Well-Being, EF; Energy/Fatigue, C; Cognitive, ME; Medication Effects, SF; Social function, OS; Overall score.