Abstract
Previously unknown phenylarsenic chemicals that originated from chemical warfare agents (CWAs) have been detected and identified in sediment samples collected from the vicinity of chemical munition dumpsites. Nontargeted screening by ultrahigh-performance liquid chromatography–high-resolution mass spectrometry (UHPLC-HRMS) was used for detection of 14 unknown CWA-related phenylarsenic chemicals. Methylated forms of Clark I/II, Adamsite, and phenyldichloroarsine were detected in all analyzed sediment samples, and their identification was based on synthesized chemicals. In addition, other previously unknown CWA-related phenylarsenic chemicals were detected, and their structures were elucidated using MS/HRMS technique. On the basis of relative isotope ratios of protonated molecules and measures of exact masses of formed fragment ions, it could be concluded that some of these unknown chemicals contained a sulfur atom attached to an arsenic atom. In addition to that, some of the samples contained chemicals that had formed via addition of an OH group to the aromatic ring. However, it is not possible to say how these chemicals are formed, but the most plausible cause is activities of marine microbes in the sediment. To our knowledge, these chemicals have not been detected from sediment samples previously. Sensitive analytical methods are needed for these novel chemicals to assess the total CWA burden in marine sediments, and this information is essential for the risk assessment.
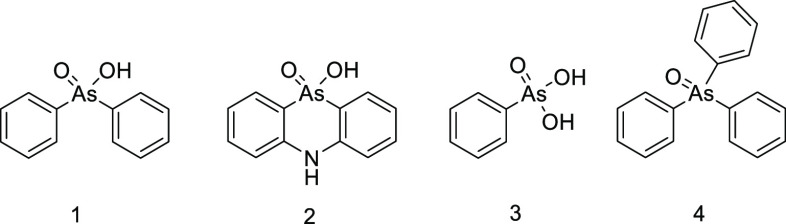
Recently, abandoned chemical weapons (CWs) containing toxic chemical warfare agents (CWAs) manufactured during the World Wars (WWs) have raised concern not only for environmental but also safety reasons. Until the 1970s, chemical munitions and containers filled with CWAs were disposed worldwide mainly by sea-dumping, and some countries were trying get rid of these toxic chemicals by burying them in the ground soil.1 Huge dumping operations took place in the Baltic Sea area and Skagerrak Strait, where the loads of dumped chemical weapons were approximately 50 000 and 170 000 tons, respectively.2 These dumping operations were adopted all around Europe; Japanese and North American coastal areas and the Pacific Ocean were also loaded with chemical munitions. It has been estimated that the total amount of chemical munitions dumped into seas and oceans is as high as 1 million tons including CWAs produced during the WWs and the postwar period.1 In the Baltic Sea area, dumped munitions contain mainly sulfur mustard and phenylarsenic CWAs, such as Clark I and II (DA and DC) and Adamsite (DM). Furthermore, technical Clark, so-called arsine oil, consisting of phenyldichloroarsine (Pfiffikus, PDCA), triphenylarsine (TPA), Clark I, and arsenic trichloride (AsCl3), was dumped. Arsine oil is a tactical mixture, and it was used as an additive in sulfur mustard to lower its freezing point. Structures of these CWA-related phenylarsenic chemicals are shown in Figure 1.
Figure 1.
Chemical structures of Clark I (DA (1)), Clark II (DC (2)), Adamsite (DM (3)), Piffikus (PDCA (4)), and triphenylarsine (TPA (5)).
Several investigations dealing with identification, determination of exact location sites, and corrosion stages of dumped warfare objects have been accomplished during the last two decades. On the basis of the information gained from several sediment-monitoring campaigns in the Baltic Sea area and Skagerrak, it has been clearly demonstrated that corroded ammunitions are leaking into the marine environment, causing risk to the marine ecosystem.1,3 Moreover, it has been proven that these CWAs are uptaken by different marine biota species.4,5
All previous sediment-monitoring campaigns in the Baltic Sea and Skagerrak areas have focused on analyzing intact CWAs and their known primary degradation products either by gas chromatography–electron impact (tandem) mass spectrometry (GC-EI/MS, GC-MS/MS) or liquid chromatography tandem mass spectrometry (LC-MS/MS).6−8 The structures of known primary degradation products of sea-dumped phenylarsenic CWAs are presented in Figure 2. These degradation products are known to be formed via hydrolysis and subsequent oxidation reactions in the water environment.6,9
Figure 2.
Primary degradation products of sea-dumped CWAs: diphenylarsininc acid (DPA (1)), phenarsazinic acid (2), phenylarsonic acid (PAA (3)), and triphenylarsine oxide (TPAO (4)).
According to the latest published study, 31% of the sediment samples collected near the CW dumping areas contained at least one of the target CWA compounds.7 For example, concentrations such as 210, 1300, and 41 μg/kg of dried sediment were detected by LC-MS/MS for phenarsazinic acid, diphenylarsinic acid (DPA), and phenylarsonic acid (PAA), respectively. Results obtained during EU BSR Project DAIMON (www.daimonproject.com) in 2019 showed that the CWA pollution level in some sampling sites in Bornholm deep was as high as 55, 2000, and 5700 μg/kg of dried sediment analyzed by LC-MS/MS for phenarsazinic acid, DPA, and PAA, respectively.10 Similar results have been obtained from the Skagerrak area: 1100 mg/kg from dried sediment for propanethiol derivatives of Clark I and PDCA analyzed by GC-EI/MS.8
The Kizaki area in Japan is one of those areas where CWs containing phenylarsenic chemicals were buried in the ground soil. In 2002 people started to get serious central nervous system symptoms after drinking contaminated well water.11 Graphite furnace atomic absorption spectrometry (AAS) analysis revealed that the arsenic level in well water was 450 times higher than the recommendations of the World Health Organization (WHO).12 Further investigations based on GC-MS and inductively coupled plasma mass spectrometry (ICP/MS) demonstrated that the source of elevated arsenic concentrations was degradation products of arsenic-containing CWAs such as DPA, PAA (see Figure 2), and bis(diphenyl)arsine oxide (BDPAO) that originated from CWs buried in the soil.12 BDPAO is a dimerized degradation product of Clark I/II.9 Soil samples taken in the Kizaki area showed contamination with these chemicals; furthermore, methylphenylarsinic acid (MPAA), dimethylphenylarsine oxide (DMPAO), and methyldiphenylarsine oxide (MDPAO) were detected.13 Structures of these methylated degradation products of CWAs are presented in Figure 3.
Figure 3.
Methylated degradation products of CWAs: methylphenylarsinic acid (MPAA (1)), dimethylphenylarsine oxide (DMPAO (2)), and methyldiphenylarsine oxide (MDPAO (3)).
It has been suggested that these methylated phenylarsenicals are formed by bacteria under anaerobic conditions.14,15 Unlike in the water environment, the behaviors of these chemicals in terrestrial environments have been studied to some extent. Transformation of DPA was investigated in the soil cultures under sulfate-reducing conditions.16 After 5-week incubation, samples were analyzed by LC-ICP/MS, showing that DPA was decomposed into arsenate, PAA, MPAA, and MDPAO. Moreover, three unknown metabolites were detected but not identified. These unknowns were eluted after DPA when the C18 column was used, suggesting that the metabolites have a more hydrophobic nature compared to DPA. A paper published a year later demonstrated the identification of the major metabolite formed in soil cultures spiked with DPA.17 Time-of-flight high-resolution mass spectrometry (TOF-HRMS) was utilized for obtaining the exact mass and proposed elemental composition for the detected degradation product. In the HRMS spectrum, a peak at m/z 260.97179 with the elemental composition of C12H12AsSO was detected. This suggested that the ion at m/z 279 is a protonated molecule ion of diphenylthioarsinic acid, but further structure elucidation of the detected metabolite was not possible by TOF-MS.
In this Article, we describe the identification of previously unknown degradation products that originated from sea-dumped CWAs based on synthesized chemicals and Orbitrap high-resolution mass spectrometry (OT-HRMS) measurements. Selected marine sediment samples were analyzed using a nontargeted screening method in order to investigate whether unknown phenylarsenic chemicals that originated from CWAs exist in the sediments. These samples were previously analyzed quantitatively for known primary degradation products of CWA-related phenylarsenic chemicals containing high concentrations of target chemicals (see Figure 2). OT-HRMS was utilized for detection and identification of unknowns. The high resolving power and high mass accuracy provided by OT-HRMS enables screening and identification of unknown chemicals without a reference standard from a complex matrix, such as marine sediment.
Thus far, the investigations have focused only on intact sea-dumped chemicals and their known primary degradation products. To estimate the total CWA burden in the sea bed, the information provided in this study is crucial. To our knowledge, there are no previous published studies on methylated, sulfur-containing, and hydroxylated degradation products of phenylarsenic CWAs in marine sediment. In addition, this is first time that these chemicals have been detected and identified in any sediment samples.
Experimental Section
Chemicals and Reagents
PDCA, DM, and DA were synthesized at Finnish Institute for Verification of the Chemical Weapons Convention. Methanol (MeOH) (LC-MS grade) and acetonitrile (ACN) (HPLC grade) used in sample pretreatment and tetrahydrofuran (THF9 (HPLC grade) were obtained from VWR International (Belgium) and Merck Group (Germany), respectively. ACN and formic acid (FA) (both LC-MS grade) were purchased from Merck, and hydrogen peroxide (33%) was obtained from VWR International. Methyl lithium (MeLi) (99%) was obtained from Sigma-Aldrich. Water was purified using a Direct-Q3 UV system (Millipore, Germany).
Sediment Samples
Samples containing high concentrations of phenylarsenic CWAs collected during the previous international projects dealing with marine munitions (CHEMSEA, MODUM, and DAIMON projects) were selected for nontargeted screening by UHPLC-HRMS. The list of the current target phenylarsenic CWA chemicals18 is presented in the Supporting Information (Table S-1), and the concentrations of target chemicals in these sediment samples are given in the Supporting Information (Table S-2). Sediment samples collected from the area where no CWA contamination has occurred were also analyzed as matrix blanks. The samples were shipped frozen on dry ice and stored at −20 °C prior to reanalysis.
Sample Preparation
Sediment samples were prepared according to “Recommended Procedures for Sampling and Analysis in Verification of Chemical Disarmament” (ROP).18 In general, sample preparation consisted of removal of pore water, extraction with ACN, filtration, and solvent exchange (H2O/MeOH, 50:50, v/v) steps. The sample pretreatment controls were done by spiking DA, DM, and TPA in blank sediment.
UHPLC-HRMS Analysis
The UHPLC-HRMS analyses were performed on Thermo Scientific Orbitrap Fusion mass spectrometer (San Jose, U.S.A.) connected to Thermo Scientific Dionex Ultimate 3000 ultrahigh-performance liquid chromatograph (Germering, Germany).
UHPLC separation was done using Waters XBridge BEH C18 column (2.1 × 50 mm, 1.7 μm) at 40 °C using a linear gradient of two mobile phases: 0.1% FA in water (A) and 0.1% FA in ACN (B). The gradient was run from 5% B at 0 min to 100% B at 2 min. After this, the B eluent was kept at 100% for 1 min and at 5% for 2 min. The flow rate was 0.5 mL/min, and the injection volume was 5 μL.
The ionization was done using a heated electrospray (HESI) probe in the positive ion mode. The instrumental parameters were set as follows: spray voltage 3000 V, source temperature 300 °C, ion transfer tube temperature 350 °C, sheath gas 30, auxiliary gas 10, and sweep gas 0. Mass measurement in full-scan mode was done with mass range m/z 60–600 using RF lens at 60% and quadrupole isolation (m/z 60–600) at a resolution of 120 000. The mass accuracy of the instrument using external calibration was specified to be ≤3 ppm. For MS/HRMS measurements, higher-energy collisional dissociation (HCD) was used to induce the fragmentation of selected protonated molecule ions.
Results and Discussion
Analysis of Sediment Samples
All samples were analyzed in full-scan mode using nontargeted analysis. An example of a total ion chromatogram (TIC) of a sediment sample is given in the Supporting Information (Figure S-1). Matrix blank samples (sediment extracts and solvent) were analyzed before and after sediment samples analysis. No cross-contamination arose from sample pretreatment, and no carryover occurred during instrumental analysis. Novel chemicals discussed in this Article were not detected in matrix blank sediment samples nor in sample pretreatment controls.
Identification of Methylated Phenylarsenic Chemicals
In full-scan mode, unknown peaks were detected in the sediment samples at m/z 199.01034, 274.02084, and 261.02562 with elemental compositions of C8H12OAs, C13H13ONAs, and C13H14OAs, respectively. This strongly suggests that the sediment samples contained DMPAO, 10-methyl-5H-phenarsazinine 10-oxide (10-M-5H-PAO), and MDPAO, respectively.
For reliable qualitative identification, methylated phenylarsenic chemicals were prepared using MeLi as a methyl group donor. Detailed synthesis procedures are described later for the synthesized chemicals. The synthesis of MDPAO is presented in Scheme 1.
Scheme 1. Synthesis Scheme for MDPAO.
Methyldiphenylarsine Oxide
Clark I (12.3 mg; 0.047 mmol) was dissolved in dry THF (0.5 mL) under argon atmosphere. MeLi (35 μL) was added through the septum, and the reaction mixture was strirred for 1 h at room temperature. The reaction was stopped by adding 0.5 mL of water.
10-Methyl-5H-phenarsazinine 10-Oxide
Adamsite (14.5 mg; 0.052 mmol) was dissolved in dry THF (0.5 mL) under argon atmosphere. MeLi (35 μL) was added through the septum, and the reaction mixture was stirred for 1 h at room temperature. The reaction was stopped by adding 0.5 mL of water.
Dimethylphenylarsine Oxide
PDCA (15.3 mg; 0.069 mmol) was dissolved in dry THF (0.5 mL) under argon atmosphere. MeLi (50 μL) was added through the septum, and the reaction mixture was stirred for 1 h at the room temperature. The reaction was stopped by adding 0.5 mL of water. The synthesis products were used as analytical standards after dilution.
The qualitative identifications of these methylated phenylarsenic compounds were based on criteria of the Organisation for the Prohibition of Chemical Weapons (OPCW).19 In LC analysis, the retention time of the identified compound shall not differ more than ±0.2 min from that of the reference standard sample (see Figure 4). For HRMS techniques, the elemental composition has to be obtained and the mass accuracy with a mass error must be below 2.5 ppm (ppm).
Figure 4.
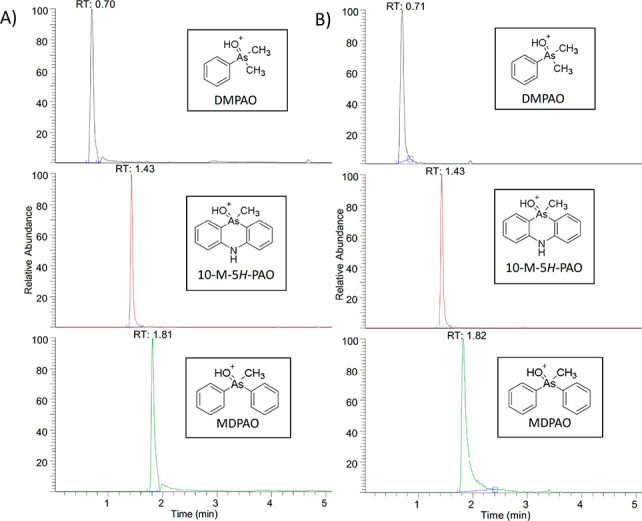
UHPLC–HESI/HRMS total ion chromatograms for DMPAO, 10-M-5H-PAO, and MDPAO in analytical standard (A) and sediment sample (B).
For further structure elucidation, HCD was used for generating fragments from protonated molecule ions of different methylated phenylarsenicals. Spectrometric parameters for different methylated phenylarsenic chemicals are presented in Table 1.
Table 1. Mass Spectrometric Parameters Used for the Detection and Characterization of Methylated Phenylarsenic Chemicals.
| compound | parent ion (m/z) | HCD (%) | scan range (m/z) |
|---|---|---|---|
| DMPAO | 199 | 50 | 70–210 |
| 10-M-5H-PAO | 274 | 40 | 70–300 |
| MDPAO | 261 | 50 | 70–280 |
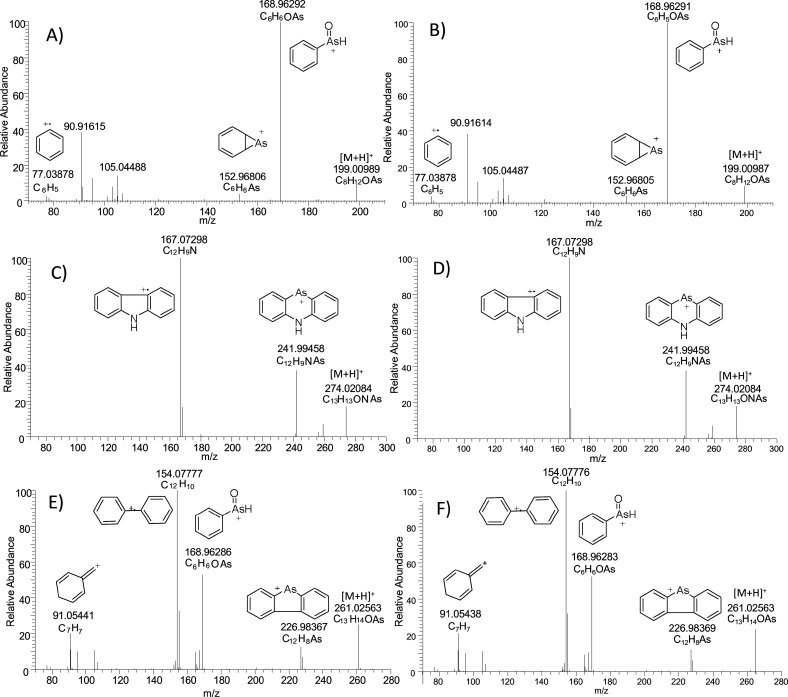
MS/HRMS spectra for different methylated phenylarsenic chemicals detected in analytical standards and sediment sample are presented in Figure 5. The MS/HRMS spectra in A, C, and E present the synthesized chemicals, and the spectra in B, D, and F are from the original sediment sample. The elemental compositions of the protonated molecules and the formed fragments were found to match with the elemental compositions of the protonated molecules and the formed fragments of the synthesized chemicals.
Figure 5.
MS/HRMS spectra for DMPAO in analytical standard (A) and sediment sample (B), 10-M-5H-PAO in analytical standard (C) and sediment sample (D), and MDPAO in analytical standard (E) and sediment sample (F).
As seen in Figure 5, fragmentation of DMPAO (A and B) generates signals at m/z 168.96291 and 152.96805, fragmentation of 10-M-5H-PAO (C and D) generates signals at m/z 241.99458 and 167.07298, and fragmentation of MDPAO (E and F) generates signals at m/z 226.98369, 168.96283, and 154.07776. These fragments are generally known to be specific for PAA, phenarsazinic acid, and DPA (see Figure 2), respectively. This makes it indisputable that these detected chemicals originated from phenylarsenic CWAs. The determination of purity and concentrations of synthesized chemicals was not enabled in the framework of this study; therefore, the quantitative analysis of methylated phenylarsenic chemicals in sediment samples was not possible. However, the peak areas of these chemicals were compared to the peak areas of primary degradation products found in the same sediment sample. Even though the ionization efficiency of analytes with different chemical properties varies, the peak areas of methylated chemicals in some sediment samples are even higher than the peak areas of current target chemicals. Data are shown in the Supporting Information (Figure S-2).
Identification of Sulfur-Containing Phenylarsenic Chemicals
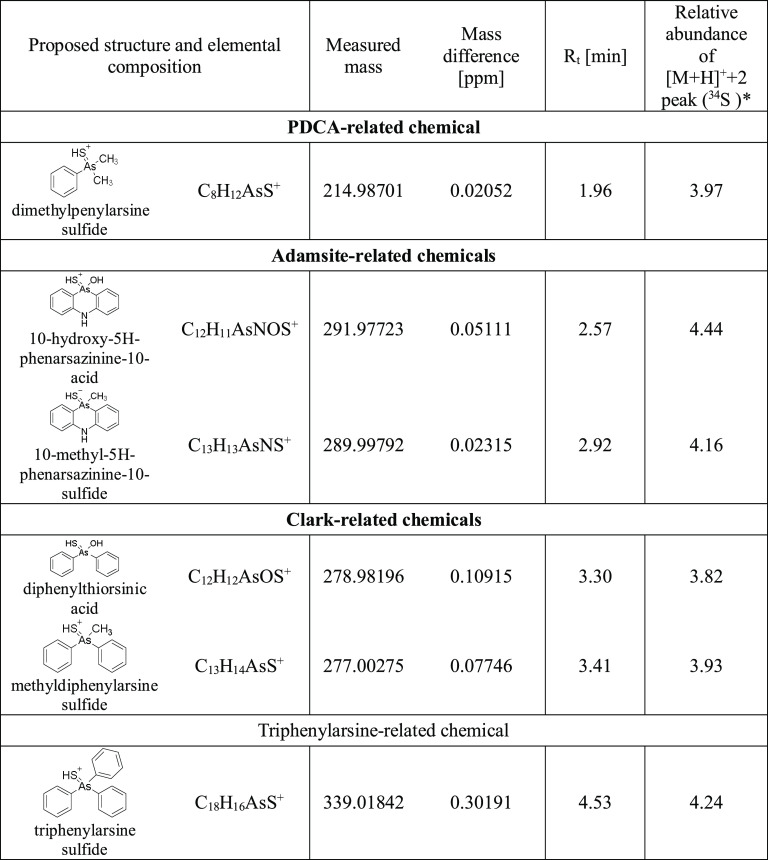
All analyzed sediment samples contained unknown chemicals that did not appear in TICs of blank sediment samples. Proposed structures for protonated molecule ions, their elemental compositions, measured masses, mass differences compared to theoretical masses, retention times, and relative abundance of isotope 34S are presented in Table 2. The presence of a distinct [M + H]+ + 2 peak for detected chemicals indicates that the structures of these chemicals contain one sulfur atom. In general, the relative abundance of the [M + H]+ + 2 peak of the detected compounds varied from 3.79 to 4.4%. Ratios of [M + H]+ and [M + H]+ + 2 peaks strongly suggest that each compound contained 32S and 34S isotopes, which is crucial for elemental composition elucidation.
Table 2. Proposed Structures of Novel Sulfur-Containing Phenylarsenic Chemicals in Sediment, Corresponding Elemental Composition, Measures Masses, Mass Differences Compared To Theoretical Values, Retention Times, and Relative Abundance of [M + H]+ + 2 Iona.
Asterisk indicates that observed interval of 34S isotope abundance in natural material is 3.96–4.77%.20
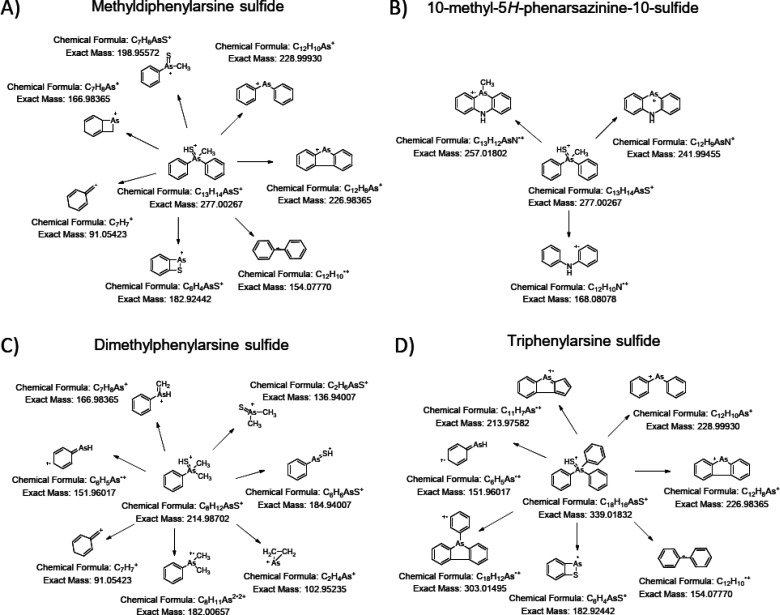
It is very likely that the polar properties of these phenylarsenic chemicals decrease when sulfur is attached to the arsenic atom as compared to their corresponding oxygen analogues (structures presented in Figure 2). This is seen in retention times: their elution out of the C18 column is delayed 1.25–1.79 min compared to their oxygen-containing analogues. The structure elucidation of these sulfur-containing chemicals was done by MS/HRMS using different HCD energies. MS/HRMS spectra were recorded, and the structures of the formed fragments were elucidated based on the measured mass. Proposed fragmentation pathways for methyldiphenylarsine sulfide, 10-methyl-5H-phenarsazinine sulfide, dimethylphenylarsine sulfide, and triphenylarsine sulfide are shown in Figure 6. Masses of the fragments presented in Figure 6 are theoretical, and they all are present in the spectra of corresponding novel compounds within a mass accuracy of 1 ppm. TICs and MS/HRMS spectra for six detected sulfur-containing chemicals (see proposed structures in Table 2) are presented in the Supporting Information (Figures S3–S7), and relative ion intensities for detected fragments are presented in the Supporting Information (Table S3).
Figure 6.
Proposed structures for fragment ions in the MS/HRMS spectra of methyldiphenylarsine sulfide (A), 10-methyl-5H-phenarsazine-10-sulfide (B), dimethylphenylarsine sulfide (C), and triphenylarsine sulfide (D).
As seen in Figure 6, compounds that have analogous structures form the same fragments. This indicates very clearly that the structures of these chemicals are similar to each other. For example, fragments with a mass at m/z 151.96017 are generated from protonated molecules of dimethylphenylarsine sulfide and triphenylarsine sulfide (see Figure 6 C and D), which have analogous structures. The same fragment is generated from DPA, a primary degradation product of DA.
This further supports the assumption that these sulfur-containing chemicals originated from CWAs. As in the case of methylated phenylarsenic chemicals, the peak areas of sulfur-containing chemicals were compared to the peak areas of the target chemicals found in the same sediment sample. Data are shown in the Supporting Information (Figures S8 and S9).
Identification of Hydroxylated Phenylarsenic Chemicals
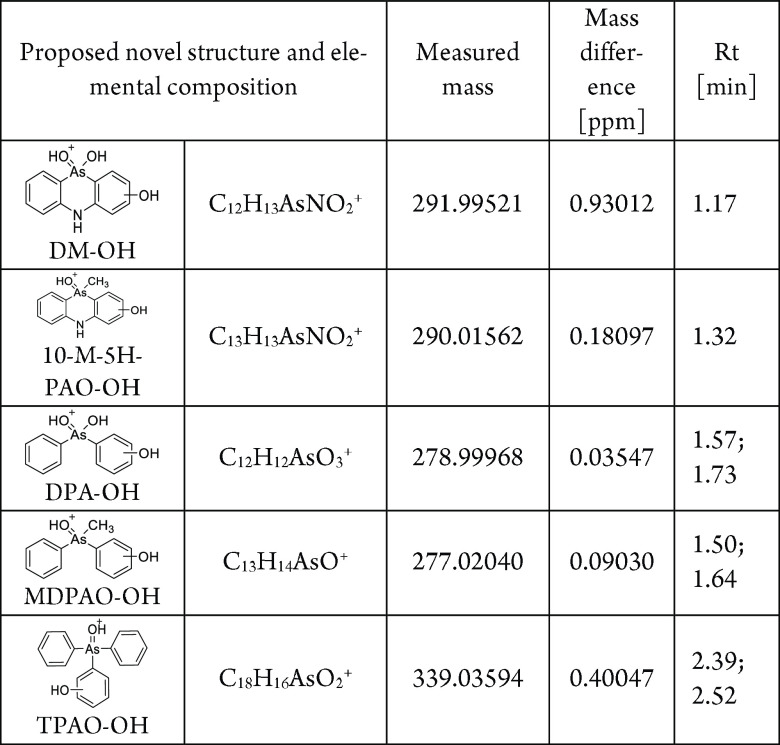
In addition to the chemicals discussed previously, five unknown degradation products were detected. On the basis of the measured masses of protonated molecules and the retention times of detected chemicals, it was assumed that the hydroxylation reactions have occurred on an aromatic ring. Proposed structures for hydroxylated phenylarsenic chemicals, corresponding elemental compositions, measures masses, mass differences compared to theoretical values, and retention times are presented in Table 3.
Table 3. Proposed Structures for Hydroxylated Phenylarsenic Chemicals, Corresponding Elemental Composition, Measures Masses, Mass Differences Compared To Theoretical Values, and Retention Times.
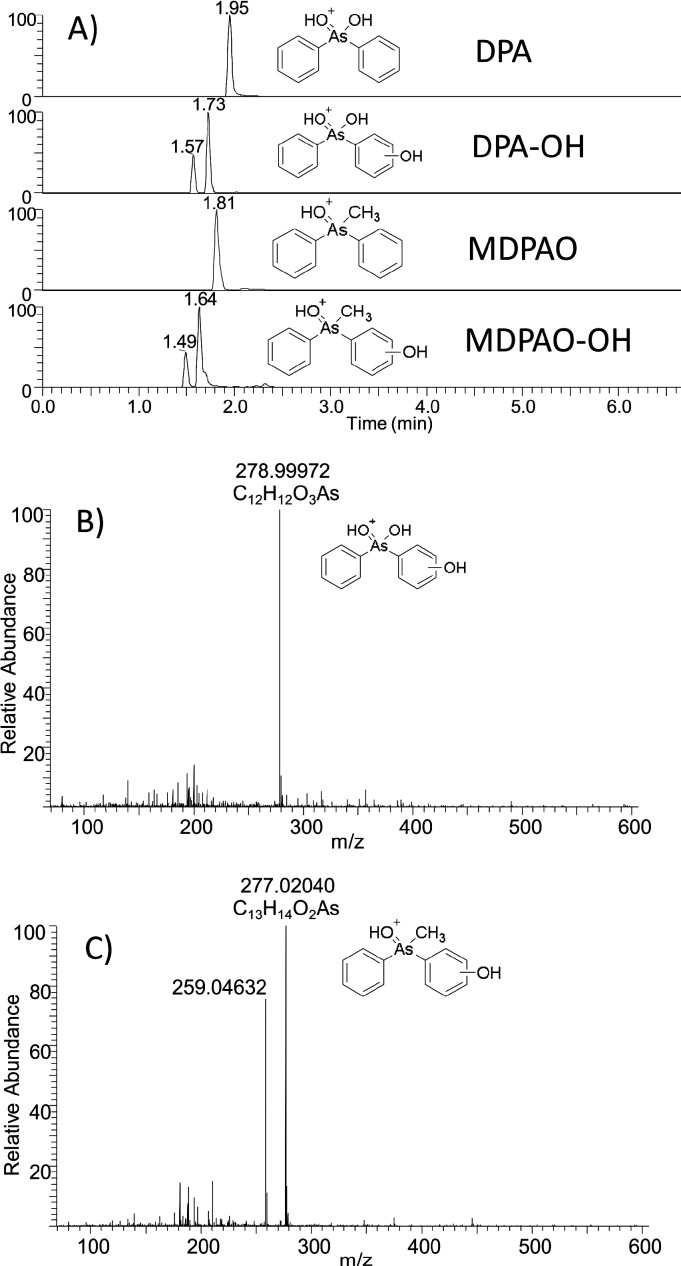
For example, DPA, an abiotic degradation of Clark I/II, and MDPAO identified in this study (see Figure 6) are seen in the mass spectra at m/z 263.00046 and 261.02562, respectively. Two Clark-type chemicals were detected with a mass difference of 16 amu compared to the protonated molecules of DPA and MDPAO, suggesting that addition of the hydroxyl group to the aromatic ring has occurred. These protonated chemicals were detected at m/z 278.99968 and 277.02040. Retention times of these chemicals are decreased compared to those of DPA and MDPAO, suggesting that these unknowns are more hydrophilic. On the basis of the measured masses, obtained elemental compositions, and retention times, these chemicals are hydroxydiphenylarsinic acid (DPA-OH) and hydroxymethyldiphenylarsine oxide (MDPAO-OH). Extracted ion chromatograms (EICs) for DPA, DPA-OH, MDPAO, and MDPAO-OH and HMRS spectra of DPA-OH and MDPAO-OH are presented in Figure 7. It is noticed that both chemicals formed two isomers where hydroxylation has occurred on different positions on the aromatic rings. This is seen in the EICs (Figure 7) where two peaks are detected at 1.57 and 1.73 min for m/z 278.99968 and 1.50 and 1.64 min for m/z 277.02040, respectively. The same phenomenon was observed with hydroxytriphenylarsine oxide (TPAO-OH), but hydroxylated forms of Adamsite-related chemicals formed only one isomer.
Figure 7.
UHPLC–HESI/HRMS EICs for hydroxylated degradation products of Clark (A) and HRMS spectra for DPA-OH (B) and MDPAO-OH (C) in sediment sample.
The more detailed structure elucidation was done by MS/HRMS for hydroxylated products of Clark- and TPA-related chemicals. HRMS spectra with identified fragments are presented in the Supporting Information (Figures S11–S13).
Conclusions
The high mass accuracy provided by OT-HRMS decreases matrix background effects, allowing reliable identification and exact molecular formula assignment for unknowns. When compared to TOF-HRMS utilized in a previously published study on identification of degradation products of CWA-related chemicals in soil samples, OT-HRMS enables more exact structure elucidation by fragmentation of protonated molecules using HCD, providing detection of fragment ions at high resolution and mass accuracy.
Detected novel phenylarsenic chemicals discussed in this Article originated from PDCA, DA, DM, and TPA, which are widely dumped at the sea bed in the Baltic Sea and Skagerrak areas. In the framework of this study, we were not able to determine the exact concentrations of the identified CWA-related chemicals discussed in this Article. By comparing the peak areas of identified phenylarsenic chemicals to the peak areas of target chemicals in the same sediment sample, we can assume that the concentration levels of methylated and sulfur-containing chemicals are at the same level or even higher. Because of the lack of information, it is not possible to say for sure how these novel chemicals are formed. Methylated degradation products identified in this study are the same as have been previously detected from CWA-contaminated soil, which strongly indicates that formation is due to microbiological activities. To prove the bacterial transformations of these phenylarsenic CWAs into the detected chemicals identified in this study, elaborate research needs to carry out. If these transformations are resulting from bacterial activities in the sediment, some, or maybe even most, of the intact phenylarsenic CWAs in the marine sediment will be transformed into methylated and sulfur-containing phenylarsenic chemicals over time, and they can further degrade into some yet unknown species.
Monitoring the sea floor quality is crucial for environmental risk assessment and further maritime spatial planning. The increasing pressure for building wind power stations, underwater pipelines, and cables requires a survey of the condition of the seabed. The previously unknown chemicals identified in this study will have a significant role in the future when analyzing concentrations of CWA-related phenylarsenic chemicals in the marine sediment. Targeted analytical methods for these novel chemicals are needed to assess the total CWA burden in marine sediments. Especially methylated and sulfur-containing degradation products of phenylarsenic CWAs should be taken into account when measuring CWA contamination levels in marine sediments.
The novel chemicals discussed in this Article might also have a hazardous impact on the ecosystem. There are no toxicity data available for these methylated and sulfur-containing phenylarsenic chemicals, and there is no knowledge on how they behave in an aqueous environment. It has already been proven that an abiotic degradation product of Clark I and/or II accumulates in fish tissues. The novel chemicals identified in this study are most likely more lipophilic than known abiotic degradation products, suggesting that these chemicals are more prone to accumulate in marine biota.
Acknowledgments
This study was performed within the framework of the EU BSR Project DAIMON (www.daimonproject.com) and was partially financed from European Regional Development Fund no. R013. The content of this publication is the sole responsibility of its authors and can in no way be taken to reflect the views of the European Union.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.9b04681.
Methods; results and discussion; list of current target phenylarsenic CWAs in sediment analysis; concentrations of current target chemicals detected in sediment samples quantitated in CHEMSEA, MODUM, and DAIMON projects; total ion chromatogram for sediment sample no. 1; extracted ion chromatograms for sediment sample no. 6 containing methylated phenylarsenicals and target chemicals; total ion chromatogram and spectrum for diphenylthioarsinic acid; total ion chromatogram and spectrum for methyldiphenylarsine sulfide; total ion chromatogram and spectrum for 10-methyl-5H-phenarsazinine-10-sulfide; total ion chromatogram and spectrum for dimethylphenylarsine sulfide; total ion chromatogram and spectrum for triphenylarsine sulfide; detected fragments and their relative abundance for all detected sulfur-containing chemicals; extracted ion chromatogram for sediment sample no. 8 containing sulfur-containing phenylarsenic chemicals and target chemicals; extracted ion chromatogram for sediment sample no. 8 containing sulfur-containing phenylarsenic chemical and target chemical; spectrum for hydroxyphenarsazinic acid; extracted ion chromatogram and spectra for two isomers of hydroxydiphenylarsinic; extracted ion chromatogram and spectra for two isomers of hydroxytriphenylarsine oxide (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Bełdowski J.; Been R.; Turmus E. K.. Towards the Monitoring of Dumped Munitions Threat (MODUM): A Study of Chemical Munitions Dumpsites in the Baltic Sea; Springer: 2017. [Google Scholar]
- Knobloch T.; Bełdowski J.; Böttcher C.; Söderström M.; Rühl N. P.; Sternheim J. Chemical Munitions Dumped in the Baltic Sea. Report of the Ad Hoc Expert Group to Update and Review the Existing Information on Dumped Chemical Munitions in the Baltic Sea (HELCOM MUNI). Balt. Sea Environ. Proc. 2013, 142. [Google Scholar]
- Bełdowski J.; Fabisiak J.; Popiel S.; Östin A.; Olsson U.; Vanninen P.; Lastumaki A.; Lang T.; Fricke N.; Brenner M.. CHEMSEA Findings; Institute of Oceanology Polish Academy of Sciences: 2014. [Google Scholar]
- Niemikoski H.; Söderström M.; Vanninen P. Detection of Chemical Warfare Agent-Related Phenylarsenic Compounds in Marine Biota Samples by LC-HESI/MS/MS. Anal. Chem. 2017, 89 (20), 11129. 10.1021/acs.analchem.7b03429. [DOI] [PubMed] [Google Scholar]
- Höher N.; Turja R.; Brenner M.; Nyholm J. R.; Östin A.; Leffler P.; Butrimavičienė L.; Baršienė J.; Halme M.; Karjalainen M.; Niemikoski H.; Vanninen P.; Broeg K.; Lehtonen K.; Berglind R. Toxic Effects of Chemical Warfare Agent Mixtures on the Mussel Mytilus Trossulus in the Baltic Sea: A Laboratory Exposure Study. Mar. Environ. Res. 2019, 145, 112–122. 10.1016/j.marenvres.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Missiaen T.; Söderström M.; Popescu I.; Vanninen P. Evaluation of a Chemical Munition Dumpsite in the Baltic Sea Based on Geophysical and Chemical Investigations. Sci. Total Environ. 2010, 408 (17), 3536–3553. 10.1016/j.scitotenv.2010.04.056. [DOI] [PubMed] [Google Scholar]
- Bełdowski J.; Klusek Z.; Szubska M.; Turja R.; Bulczak A. I.; Rak D.; Brenner M.; Lang T.; Kotwicki L.; Grzelak K.; Jakacki J.; Fricke N.; Östin A.; Olsson U.; Fabisiak J.; Garnaga G.; Rattfelt Nyholm J.; Majewski P.; Broeg K.; Söderström M.; Vanninen P.; Popiel S.; Nawala J.; Lehtonen K.; Berglind R.; Schmidt B. Chemical Munitions Search & Assessment—An Evaluation of the Dumped Munitions Problem in the Baltic Sea. Deep Sea Res., Part II 2016, 128, 85–95. 10.1016/j.dsr2.2015.01.017. [DOI] [Google Scholar]
- Tørnes J. A.; Opstad A. M.; Johnsen B. A. Determination of Organoarsenic Warfare Agents in Sediment Samples from Skagerrak by Gas Chromatography-Mass Spectrometry. Sci. Total Environ. 2006, 356 (1–3), 235–246. 10.1016/j.scitotenv.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Haas R.; Schmidt T. C.; Steinbach K.; Von Löw E. Chromatographic Determination of Phenylarsenic Compounds. Fresenius. Fresenius' J. Anal. Chem. 1998, 361 (3), 313–318. 10.1007/s002160050892. [DOI] [Google Scholar]
- Lignell H.; Karjalainen M.; Aalto S.; Taure T.; Tuomala N.; Vanninen P.. DAIMON Report on Chemical Analysis of Sea-Dumped Chemical Warfare Agents in Sediment Samples; DAIMON: 2019.
- Ishii K.; Tamaoka A.; Otsuka F.; Iwasaki N.; Shin K.; Matsui A.; Endo G.; Kumagai Y.; Ishii T.; Shoji S.; et al. Diphenylarsinic Acid Poisoning from Chemical Weapons in Kamisu, Japan. Ann. Neurol. 2004, 56 (5), 741–745. 10.1002/ana.20290. [DOI] [PubMed] [Google Scholar]
- Ishizaki M.; Yanaoka T.; Nakamura M.; Hakuta T.; Ueno S.; Komuro M.; Shibata M.; Kitamura T.; Honda A.; Doy M.; Ishii K.; Tamaoka A.; Shimojo N.; Ogata T.; Nagasawa E.; Hanaoka S. Detection of Bis(Diphenylarsine)Oxide, Diphenylarsinic Acid and Phenylarsonic Acid, Compounds Probably Derived from Chemical Warfare Agents, in Drinking Well Water. J. Health Sci. 2005, 51 (2), 130–137. 10.1248/jhs.51.130. [DOI] [Google Scholar]
- Baba K.; Arao T.; Maejima Y.; Watanabe E.; Eun H.; Ishizaka M. Arsenic Speciation in Rice and Soil Containing Related Compounds of Chemical Warfare Agents. Anal. Chem. 2008, 80 (15), 5768–5775. 10.1021/ac8002984. [DOI] [PubMed] [Google Scholar]
- Arao T.; Maejima Y.; Baba K. Uptake of Aromatic Arsenicals from Soil Contaminated with Diphenylarsinic Acid by Rice. Environ. Sci. Technol. 2009, 43 (4), 1097–1101. 10.1021/es8023397. [DOI] [PubMed] [Google Scholar]
- Maejima Y.; Arao T.; Baba K. Transformation of Diphenylarsinic Acid in Agricultural Soils. J. Environ. Qual. 2011, 40 (1), 76. 10.2134/jeq2009.0496. [DOI] [PubMed] [Google Scholar]
- Guan L.; Hisatomi S.; Fujii K.; Nonaka M.; Harada N. Enhanced Transformation of Diphenylarsinic Acid in Soil under Sulfate-Reducing Conditions. J. Hazard. Mater. 2012, 241–242, 355–362. 10.1016/j.jhazmat.2012.09.054. [DOI] [PubMed] [Google Scholar]
- Hisatomi S.; Guan L.; Nakajima M.; Fujii K.; Nonaka M.; Harada N. Formation of Diphenylthioarsinic Acid from Diphenylarsinic Acid under Anaerobic Sulfate-Reducing Soil Conditions. J. Hazard. Mater. 2013, 262, 25–30. 10.1016/j.jhazmat.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Söderström M.; Östin A.. Analysis of Sediment Samples for Sea-Dumped Chemical Weapons. Recommended operating procedures for analysis in the verification of chemical disarmament; University of Helsinki: 2017; pp 631–649. [Google Scholar]
- Organisation for the Prohibition of Chemical Weapons (OPCW) . Work Instruction for the Reporting of the Results of the OPCW Biomedical Proficiency Test; OPCW: 2018.
- Meija J.; Coplen T. B.; Berglund M.; Brand W. A.; De Bièvre P.; Gröning M.; Holden N. E.; Irrgeher J.; Loss R. D.; Walczyk T.; Prohaska T. Isotopic Compositions of the Elements 2013 (IUPAC Technical Report). Pure Appl. Chem. 2016, 88 (3), 293–306. 10.1515/pac-2015-0503. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.