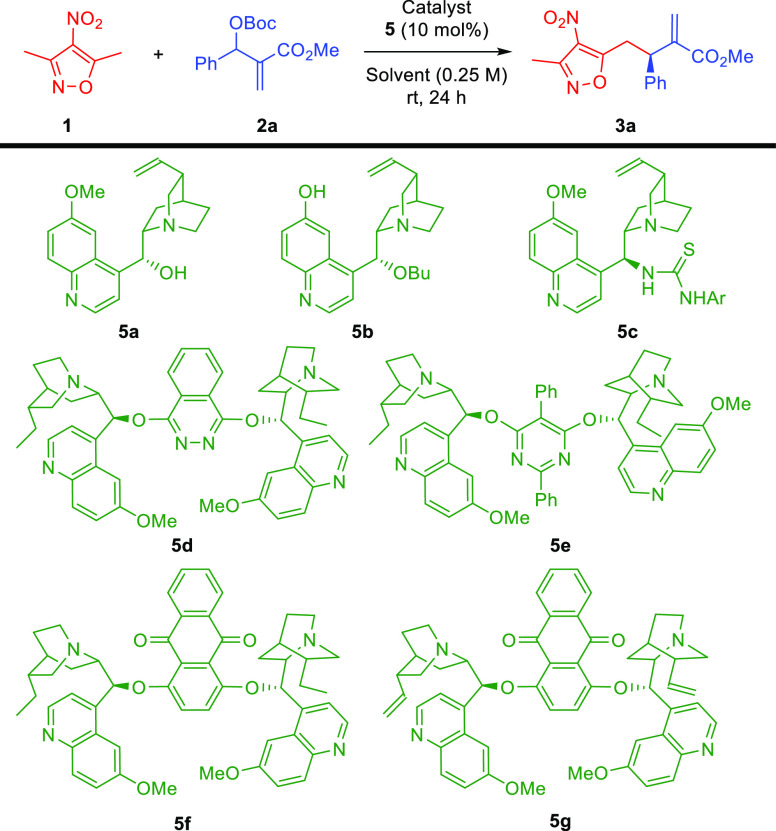
Table 1. Enantioselective Allylic–allylic Alkylation Using 3,5-Dimethyl-4-nitroisoxazole 1 as a Vinylogous Pronucleophile: Optimization Studiesa.
| entry | solvent (catalyst) | T [°C] | yield [%]b | erc |
|---|---|---|---|---|
| 1 | CH2Cl2 (5a) | rt | 26 | 60:40 |
| 2 | CH2Cl2(5b) | rt | 22 | 81:19 |
| 3 | CH2Cl2 (5c) | rt | <5 | nd |
| 4 | CH2Cl2 (5d) | rt | 22 | 79:21 |
| 5 | CH2Cl2 (5e) | rt | 41 | 88:12 |
| 6 | CH2Cl2 (5f) | rt | 43 | 90:10 |
| 7 | CH2Cl2 (5g) | rt | 38 | 76:24 |
| 8 | CHCl3 (5f) | rt | 69 | 91:9 |
| 9 | ClCH2CH2Cl (5f) | rt | 40 | 86:14 |
| 10 | toluene (5f) | rt | 19 | 89:11 |
| 11 | THF (5f) | rt | 36 | 87:13 |
| 12 | CH3CN (5f) | rt | 80 | 89:11 |
| 13d | CHCl3 (5f) | rt | 41 | 89:11 |
| 14e | CHCl3 (5f) | rt | 66 | 90:10 |
| 15 | CHCl3 (5f) | 10 | 20 | 92:8 |
| 16 | CHCl3 (5f) | 40 | 84 | 96:4 |
| 17 | CHCl3 (5f) | 60 | 78 | 90:10 |
| 18f | CHCl3 (5f) | 40 | 81 | 91:9 |
| 19g | CHCl3 (5f) | 40 | 79 | 93:7 |
Reactions performed on a 0.1 mmol scale using 1 (1 equiv) and 2a (1 equiv) in 0.4 mL of the solvent.
Isolated yields are given.
Determined by a chiral stationary phase HPLC.
Reaction was performed in 0.8 mL of CH2Cl2.
Reaction was performed in 0.2 mL of CH2Cl2.
Reaction was performed using 1 (1.5 equiv).
Reaction was performed using 2a (1.5 equiv).