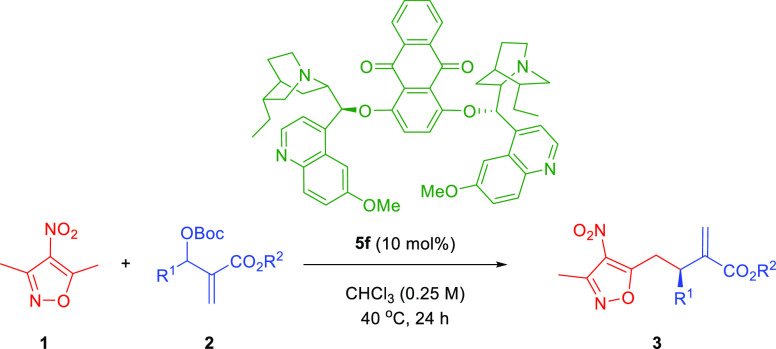
Table 2. Enantioselective Allylic–Allylic Alkylation Using 3,5-Dimethyl-4-nitroisoxazole 1 as a Vinylogous Pronucleophile: Scope Studiesa.
| entry | R1 | R2 | yield [%]b | erc |
|---|---|---|---|---|
| 1 | Ph (3a) | Me | 84 | 96:4 |
| 2 | 4-CF3C6H4 (3b) | Me | 82 | 90:10 |
| 3 | 4-BrC6H4 (3c) | Me | 86 | 97:3 |
| 4 | 4-ClC6H4 (3d) | Me | 90 | 98:2 |
| 5 | 3-ClC6H4 (3e) | Me | 89 | 96:4 |
| 6 | 2-ClC6H4 (3f) | Me | 83 | 94:6 |
| 7 | 4-CH3C6H4 (3g) | Me | 92 | 97:3 |
| 8 | 4-CH3OC6H4 (3h) | Me | 90 | 96:4 |
| 9 | 3,4-(OCH2O)C6H3 (3i) | Me | 89 | 96:4 |
| 10 | 2-furyl (3j) | Me | 84 | 98:2 |
| 11 | E-Prop-1-enyl (3k) | Me | 84 | 95:5 |
| 12 | Ph (3l) | Et | 85 | 98:2 |
| 13 | Ph (3m) | tBu | 90 | 99:1 |
| 14d | Ph (3a) | Me | 58 | 96:4 |
Reactions performed on a 0.1 mmol scale using 1 (1 equiv) and 2a (1 equiv) in 0.4 mL of the solvent.
Isolated yields are given.
Determined by a chiral stationary phase HPLC.
Reaction was performed on a 5 mmol scale.