Figure 1 |. Inhibition of the unfolded protein response contributes to heart failure with preserved ejection fraction.
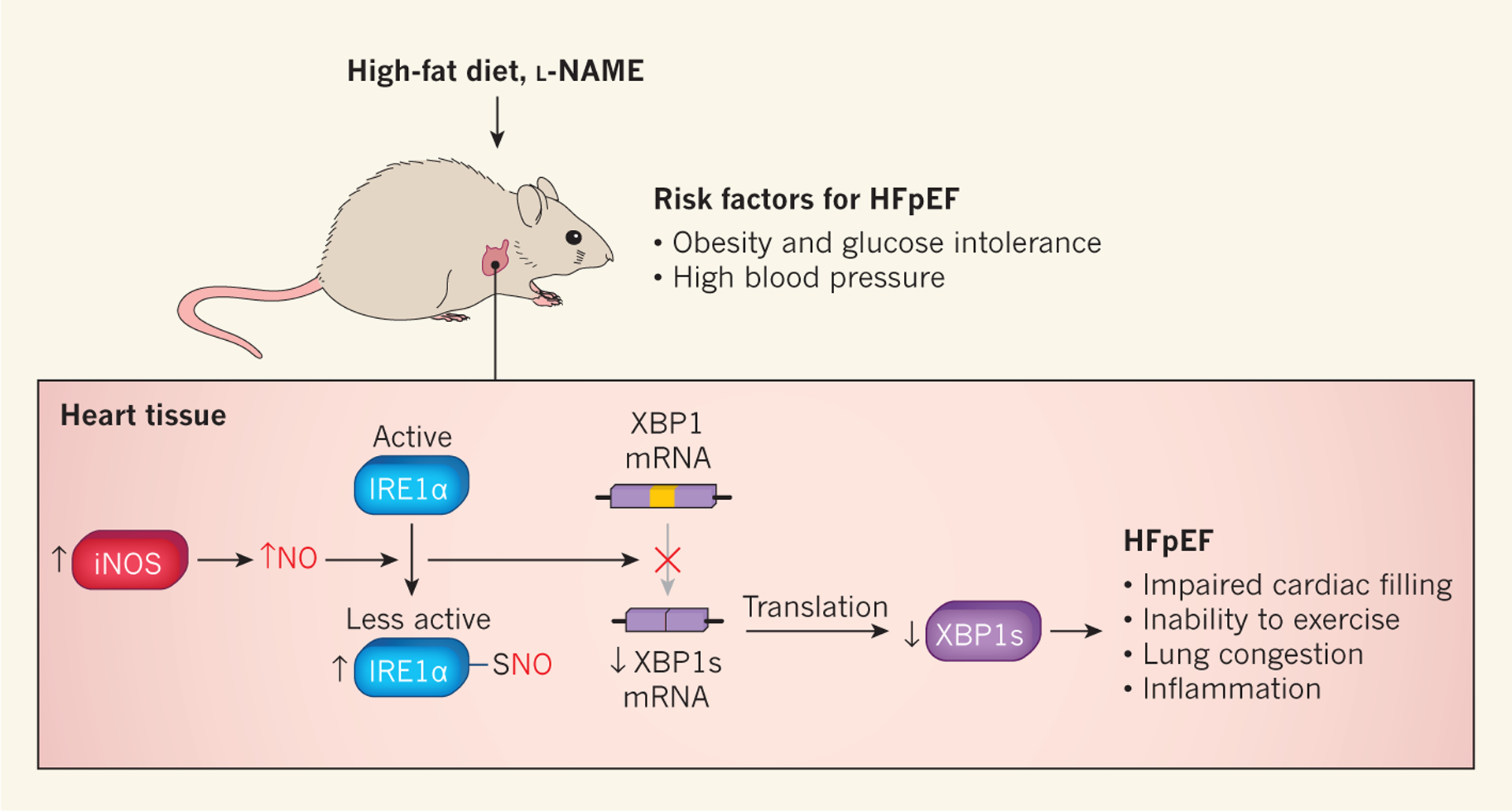
Schiattarella et al.2 fed mice a high-fat diet to produce obesity and glucose intolerance (an inability to transport sugar efficiently from the blood into cells, which can lead to diabetes), and gave the animals the drug Nω-nitro-l-arginine methyl ester (l-NAME) to cause high blood pressure. These treatments induced some of the hallmarks of heart failure with preserved ejection fraction (HFpEF), including impaired filling of the heart with blood, inability to exercise, lung congestion and increased levels of molecular markers associated with inflammation. The authors also observed increased expression of the enzyme inducible nitric oxide synthase (iNOS), which increases the production of nitric oxide (NO). The addition of NO to sulfur atoms (S-nitrosylation) of inositol-requiring enzyme 1α (IRE1α) decreases this protein’s activity. IRE1α is a component of the unfolded protein response (UPR), a mechanism that protects cells against abnormal levels of misfolded proteins. Decreased IRE1α activity leads to reduced conversion (splicing) of messenger RNA encoding X-box binding protein 1 (XBP1) to the mRNA that encodes XBP1 spliced (XBP1s), a transcription factor that activates UPR genes. The authors observed that IREα splicing activity and XBP1s levels were reduced in their mouse model of HFpEF.
