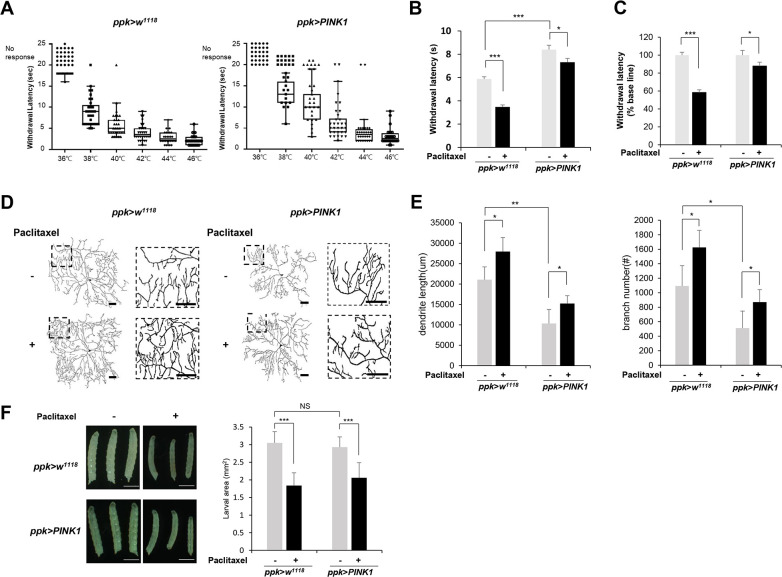
Fig 2. PINK1 mitigates the heat-hyperalgesia phenotype induced by paclitaxel treatment.
(A) The thermal nociceptive response of ppk-GAL4 control L3 larvae (ppk>w1118) and larvae expressing PINK1 in C4da sensory neuron (ppk>PINK1) to heat probes at different temperatures. Each data point represents the withdrawal latency of an individual larva. The absence of an aversive rolling response within 20 sec was considered no response. n = 50 larvae were tested at each temperature. (B) Thermal nociceptive withdrawal of ppk>w1118 and ppk>PINK1 larvae from heat probe (40°C) after 48 h of exposure to either vehicle (DMSO) or 20 μM paclitaxel (n = 50 per sample). (C) The relative withdrawal latency of ppk>w1118 and ppk>PINK1 larvae upon paclitaxel treatment was calculated according to that of the vehicle sample of each genotype, which was considered to be 100%. (D) Representative images of C4da neurons at abdominal segment A4 of control L3 larvae (ppk1a > CD4-tdTom) and L3 larvae expressing PINK1 (ppk1a > CD4-tdTom,PINK1). Larvae were treated with paclitaxel (20 μM) for 48 h. Right images are enlargements of the boxed regions in the left images. Scale bars, 50 μm. (E) Quantification of the length of dendrites (left) and the number of dendrite branch points (right) in C4da neurons. n = 5 per sample. (F) Representative images of larvae from each genotype at 120 AEL treated with either DMSO or paclitaxel for 48 h (left). The larval areas calculated by the length multiplied by the width of each larva of each genotype after either DMSO or paclitaxel treatment were plotted (n≧50 per sample) (right). Scale bars, 1 mm. The results are presented as the mean values, and the error bars represent the SD. Significance was determined by one-way ANOVA with Sidák correction. *P <0.05; **P <0.01; ***P <0.001. NS; not significant.