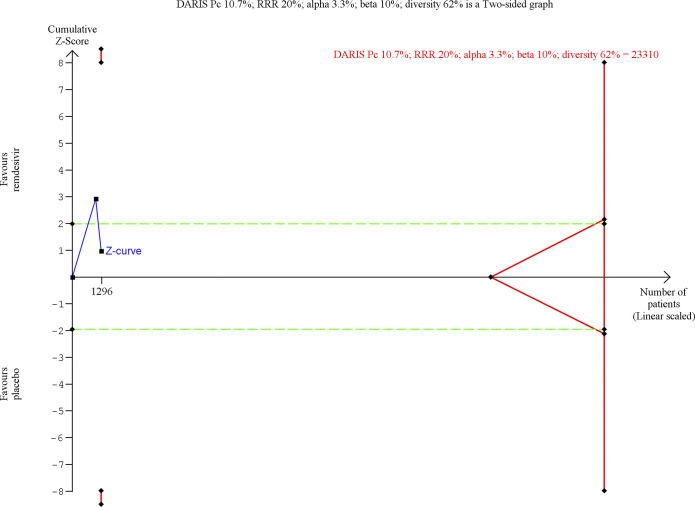
Fig 3. Trial sequential analysis of remdesivir versus placebo on all-cause mortality.
Trial sequential analysis on remdesivir versus placebo on all-cause mortality in 2 trials at high risk of bias. The DARIS was calculated based on a mortality proportion in the control group of 10.7%; risk ratio reduction of 20% in the experimental group; type I error of 3.3%; and type II error of 10% (90% power). Diversity was 62%. The required information size was 23,310 participants. The cumulative Z‐curve (blue line) did not cross the trial sequential monitoring boundaries for benefit or harm. The cumulative Z‐curve did not cross the inner‐wedge futility line (red outward sloping lines nor the DARIS). The green dotted line shows conventional boundaries (alpha 5%). DARIS, diversity‐adjusted required information size; Pc, proportion of participants in control group; RRR, relative risk reduction.