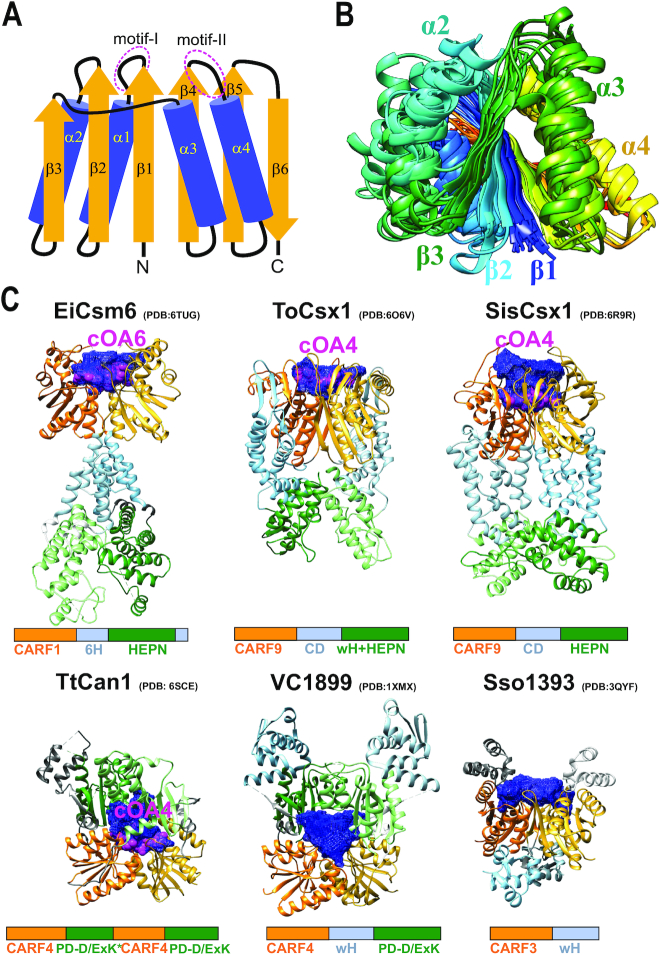
Figure 3.
Structures of the CARF domain containing proteins. (A) Schematic representation of the conserved core of the CARF fold. Motifs I and II (corresponding to β1-α1 and β4-α4 junctions, respectively) are involved in cyclic oligoadenylate binding/cleavage activities. (B) Superimposed structures of 11 CARF domains colored according to chain progression from N-terminus (blue) to C-terminus (red). Non-conserved loops/insertions were removed for clarity. (C) Selected structures of CARF proteins with different domain architectures. Domain homodimers (different chains) or heterodimers (single chain as in TtCan1) are represented by different shades of the same color. All CARF domain dimers have a cleft (blue mesh) in the corresponding structural regions. This cleft (pocket) is where a cyclic oligoadenylate (shown in pink and labeled) binds. CARF domains, orange; domains in topologically equivalent positions following CARF (Csx1 connector domain, Csm6 6H domain and wHTH domain), light blue; toxin domains (HEPN, wHTH-HEPN and PD-D/ExK), green.