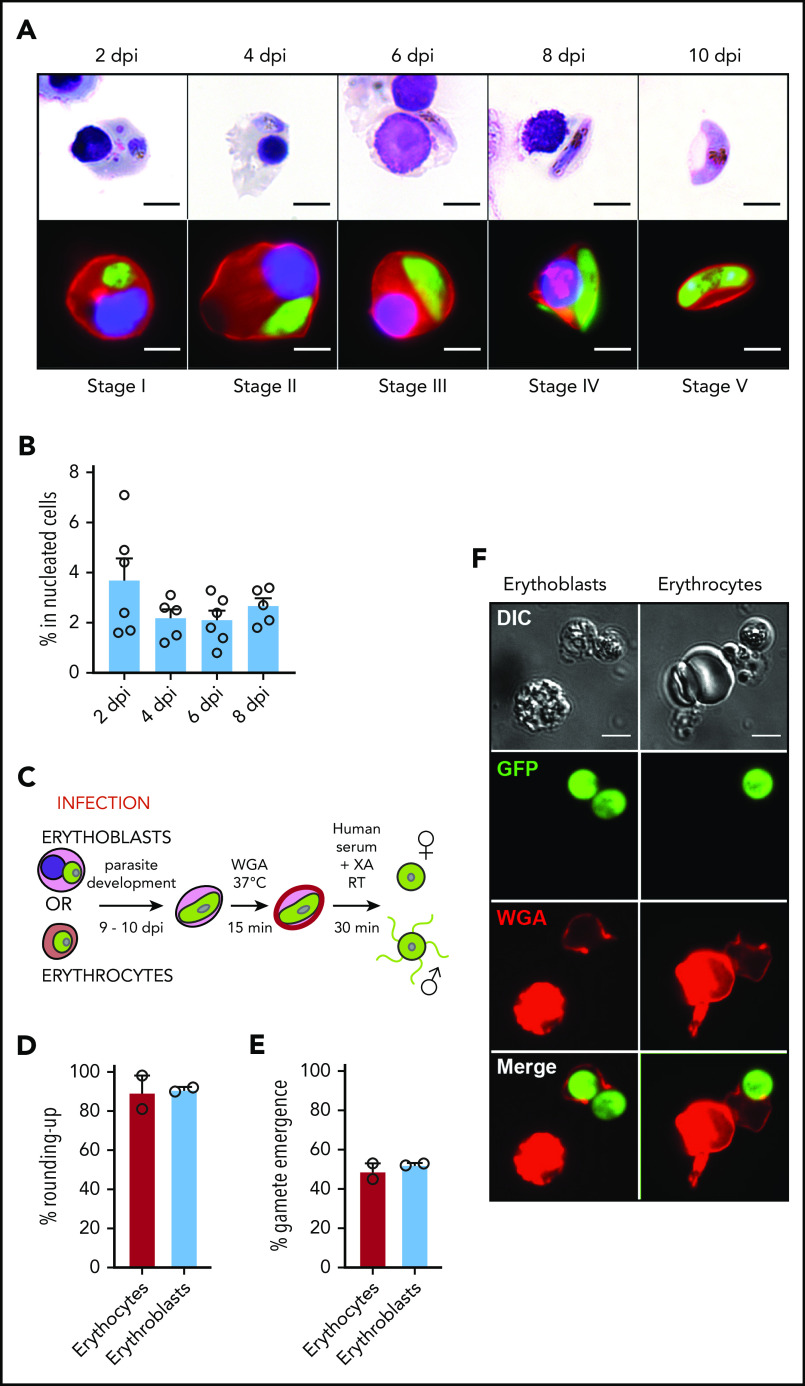
Figure 2.
Infection of erythroblasts allows complete gametocytogenesis. (A) Gametocyte maturation after erythroblast infection with the Pfs16-GFP line was observed by light microscopy after May-Grünwald Giemsa staining (upper panels) and by fluorescent microscopy (lower panels). Erythroblast membrane was stained with PKH-26 (red). DNA was stained with Hoechst 33342 (blue). Scale bars, 5 μm. (B) Infection of erythroblasts with the Pfs16-GFP line was evaluated by flow cytometry at 2, 4, 6, and 8 dpi. Circles indicate the number of independent experiments (n = 6) that were performed on erythroblasts derived from cytaphereses (n = 5) or bone marrow aspirates (n = 1) from 4 independent donors. (C) Diagram illustrating the gamete activation protocol. After infection of erythroblasts or erythrocytes with Pfs16-GFP schizonts, gametocytes were allowed to differentiate for 9 to 10 days within erythroid precursors or erythrocytes. Mature gametocytes were stained with WGA–Alexa Fluor 647 (red) for 15 minutes at 37°C and then activated in human serum and xanthurenic acid (XA) at room temperature (RT) for 30 minutes, leading to gamete activation. (D) GFP+ cells were scored as round or crescent shaped and plotted as percentage rounded-up. (E) GFP+ cells were scored as positive or negative for WGA-Alexa Fluor 647 staining and plotted as percentage gamete emergence. (D-E) One hundred cells were scored per condition. Circles indicate the number of independent experiments, and error bars show the standard error of the mean. (F) Fluorescence microscopy of activated gametes from a culture of infected erythroblasts (left panels) or infected erythrocytes (right panels) with the Pfs16-GFP line. Erythroid cell membrane is stained with WGA–Alexa Fluor 647 (red). Scale bars, 5 μm. DIC, differential interference contrast.