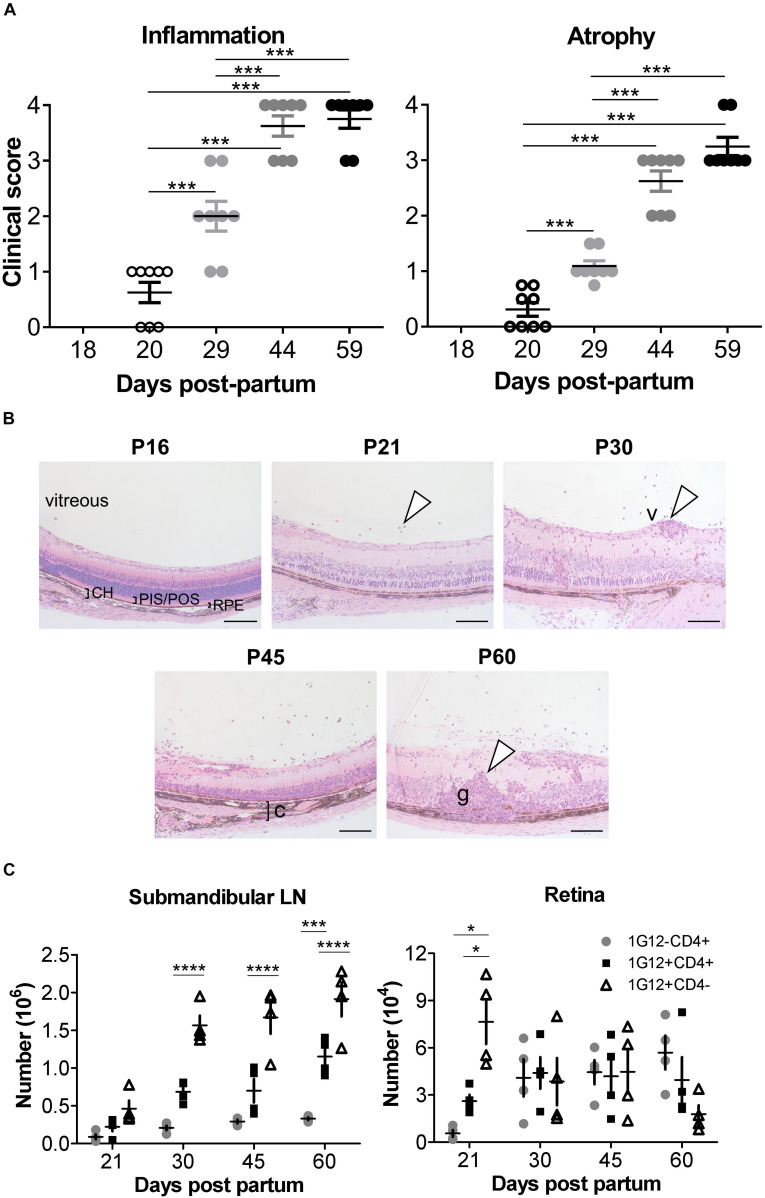
FIGURE 1.
Clinical signs and phenotypic features of ocular inflammation in dTg HEL/TCR mice. (A) Retinal inflammation and atrophy were scored separately according to Supplementary Tables 1, 2, respectively, from post-partum day P20 to P59 (n = 8/group). Chronologically, atrophic changes followed the inflammatory signs. Data were analyzed using one-way ANOVA and Tukey’s Multiple Comparison post hoc Test with ***p < 0.001 when compared with P20 controls on a 95% level of confidence. (B) Eyes from dTg mice aged from P16 to P60 were fixed in 2.5% glutaraldehyde, embedded in resin, sectioned, and stained by hematoxylin and eosin (H&E). From P21, infiltrating cells were seen in the vitreous (arrowhead). From P30 to P60, vasculitis (v,arrowhead), choroiditis (c,bracket), and granuloma (g,arrowhead) were observed. CH, choroid; PIS/POS, photoreceptor inner-/outer layer; RPE, retinal pigment epithelium. Photos were taken using a ProgRes XT Core 5 color digital microscope camera (JENOPTIK Optical Systems GmbH, Jena, Germany) and mounted on a Zeiss Axioskop 40 microscope (Carl Zeiss, MicroImaging GmbH, Jena, GE). Scale bar: 100 μm. Representative images are shown. (C) Absolute number of cells found in the submandibular/eye-draining lymph nodes (LN) and the retina, respectively, during the course of EAU in dTg mice are shown (n = 4/age group). The numbers of 1G12+ double negative (DN), 1G12+CD4+ and non-antigen-specific CD4+ T cells increased steadily with age in dTg mice. The first cells populating the eye-draining LN and retinas of dTg mice were mostly 1G12+DN. The numbers of 1G12+DN cells decreased with age in the retinas of dTg mice, whereas non-antigen-specific (naïve) CD4+ increased in the later stage of EAU. Data were analyzed using one-way ANOVA and Tukey’s Multiple Comparison post hoc Test with *p < 0.05, ***p < 0.001, ****p < 0.0001 on a 95% level of confidence. Cell numbers are the average of 4 pairs of retina or DLN, i.e., cell count/pair. 1 × 105 total events were recorded. Total numbers provided were extrapolated based on total cell counts, determined using a Coulter cell counter prior to sample processing.