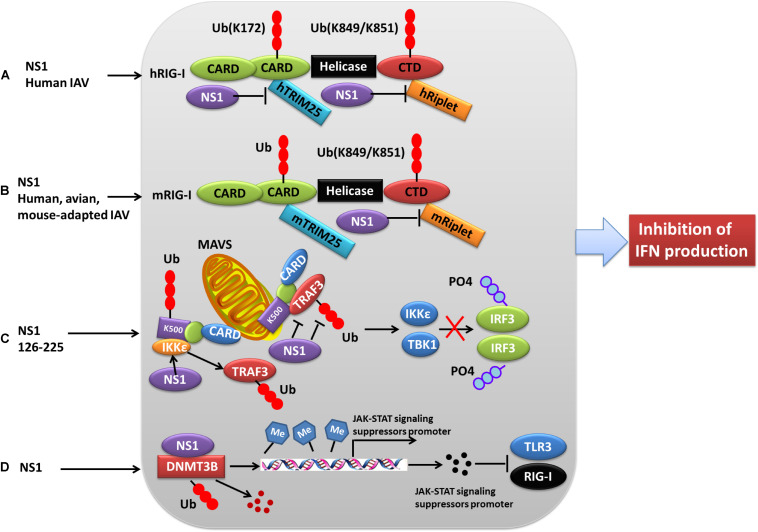
FIGURE 3.
Role of PTMs in NS1 antagonism of host interferon responses. (A) NS1 from human IAV strains can interact with human TRIM25 and Riplet, blocking them from mediating the ubiquitination of the vRNA sensor RIG-I CARD, and this eventually leads to the inhibition of IFN production. (B) NS1 proteins from avian, human, and mouse-adapted influenza viruses can inhibit RIG-I signaling in mouse cells only through binding to and blocking mouse Riplet. (C) The NS1 C-terminal effector domain (NS1 126–225) associates with TRAF3, promoting the disassociation of TRAF3 from MAVS and leading to a decreased level of K63-linked ubiquitination of TRAF3. Moreover, NS1 126–225 facilitates the recruitment of IKKε to MAVS, resulting in TRAF3 release from the mitochondria and impaired IRF3 phosphorylation. (D) NS1 interacts with cellular DNMT3B, and the resulting NS1-DNMTcomplex is translocated from the nucleus to the cytosol where it undergoes K48-linked polyubiquitination of DNMT3B. This leads to demethylation of the methylated promoters of JAK-STAT signaling suppressors and the subsequent expression of associated proteins, ultimately resulting in the inhibition of TLR3 and RIG-I. Ub, ubiquitin and ubiquitination; PO4, phosphorylation; TRIM, tripartite motif; RIG-I, retinoic acid-induced gene 1 protein; CARD, caspase recruitment domain; DNMT3B, DNA methyltransferase; TRAF3, TNF receptor-associated factor 3.