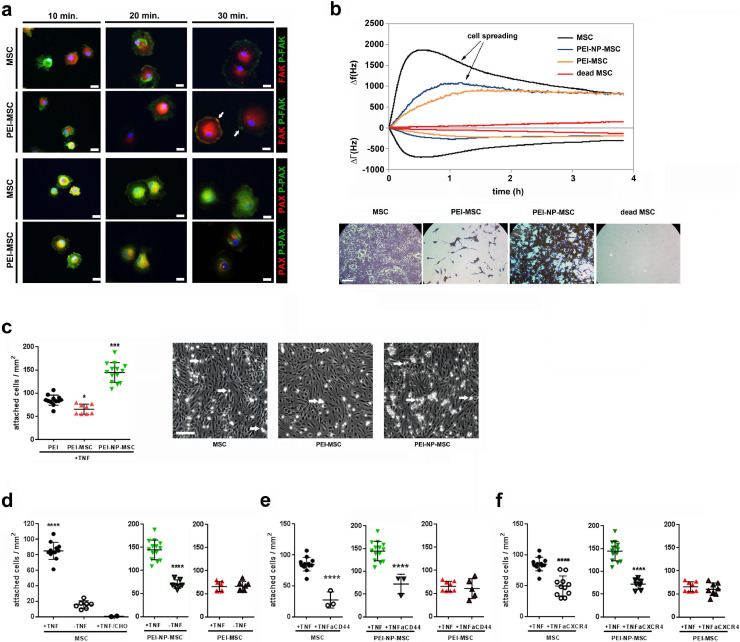
Fig. 2.
Functional in vitro adhesion capacity of human MSC after PEI treatment
(a) In vitro integrin signalling analysis by immunocytochemistry of phosphorylated (green) and non-phosphorylated (red) focal adhesion point markers, focal adhesion kinase (FAK) and paxillin (PAX). PEI-MSC showed accelerated integrin signalling and focal adhesion points clustering differed over time between treated and untreated MSC. The focal adhesion points (arrows) of PEI-MSC appeared more evenly distributed and less accentuated. Scale bars: 20 µm. Microphotographs represent two technical replicates of pooled hBM-MSC (3 donors) per group out of a single experiment.
(b) Quartz Crystal Microbalance with Dissipation (QCM-D) experiments to evaluate seeding and spreading capacities of MSC upon PEI/NP treatment in real time. QCM-D measures surface adsorption by means of shifts in the resonance frequency (Δf) and damping behaviour (bandwidth, ΔΓ) of an AT-cut quartz crystal. While a negative frequency shift is related to the amount of adsorbed mass, damping is the result of dissipative energy losses induced by the adsorbed mass. The relation between frequency and bandwidth represents the mass-sensor coupling, and a loosely coupled mass dissipates more energy (larger ΔΓ) than a firmly coupled mass (smaller ΔΓ). The figure displays representative graphs of three separate measurements for each condition. QCM-D data correlate with the microscopic images (crystal violet staining) taken after the mass sensitive measurements. Seeding density: 40,000 cells per sensor. Viable MSC can be distinguished from dead cells (negative control; 10 min. 57 °C; red graphs, right microphotograph in lower row) showing absence of significant frequency and bandwidth shifts; scale bar: 200 μm. Untreated viable MSC (black graphs) adhered within 30 minu with subsequent typical spreading signal (gradual decrease in bandwidth) in the following 4 hours. In contrast, PEI-MSC (yellow graphs) displayed slower adhesion and almost no spreading (stable bandwidth), indicating weaker adhesion. PEI-NP-MSC (blue graphs) also displayed slow adhesion, but typical spreading, although delayed and less pronounced than in MSC.
(c) Adhesion capacity of MSC and PEI/NP treated MSC under dynamic shear stress conditions to endothelial cells that were activated by TNFα. Flow chamber adhesion data of untreated and PEI/NP treated MSC were compared with ANOVA followed by Tukey's multiple comparisons test (*p < 0.05; ***p < 0.0001). Error bars: SD. Arrows indicate exemplary adherent MSC; scale bar: 200 μm. N = 8–14, each data point represents the mean of cell counts of 5–12 individual experiments per group.
(d) Adhesion capacity of MSC and PEI/NP treated MSC under dynamic shear stress conditions to endothelial cells with or without TNFα activation as well as to CHO cells lacking intact adhesion molecules. Flow chamber adhesion data of untreated MSC with or without TNFα activation and to CHO cells were compared with ANOVA followed by Tukey's multiple comparisons test (****p < 0.001); Flow chamber adhesion data of PEI/NP treated MSC with or without TNFα activation were compared with two-tailed t-test (****p<0.0001). Error bars: SD. N = 2 –12, each data point represents the mean of cell counts of 1 –8 individual experiments per group.
(e), (f) Adhesion capacity of MSC and PEI/NP treated MSC under dynamic shear stress conditions to endothelial cells with TNFα activation and blocking antibodies against CD44 or CXCR4. Flow chamber adhesion data of untreated and PEI/NP treated MSC with or without blocking antibodies were compared with two-tailed t-test (****p < 0.0001). Error bars: SD. N = 3-14, each data point represents the mean of cell counts of 2 –9 individual experiments per group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)