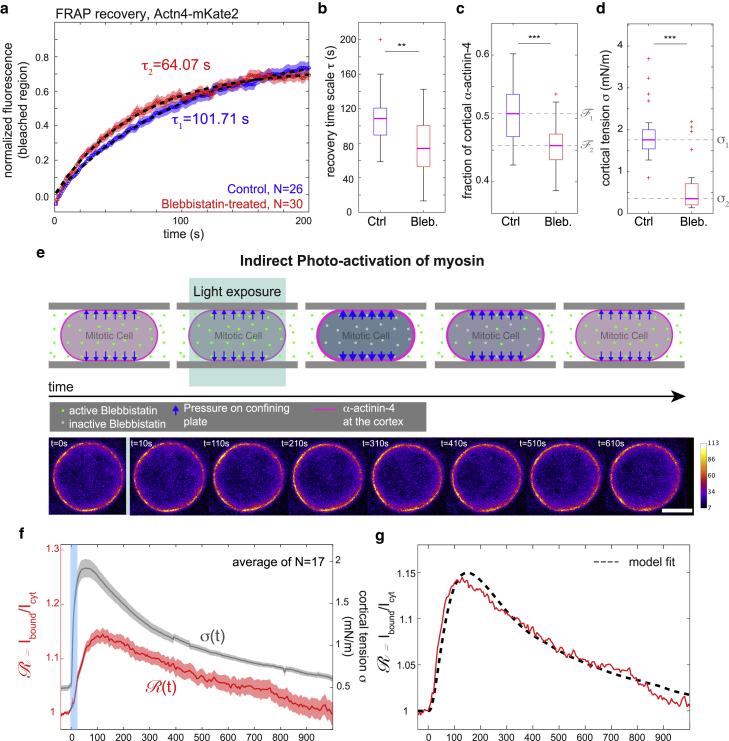
Figure 5.
Experimental results from FRAP and indirect photoactivation of myosin with red fluorescently labeled α-actinin-4 (Actn4-mKate2) in mitotic HeLa cells. (a) Averaged recovery curves of α-actinin-4-mKate2 after photobleaching at the mitotic cortex: control (N = 26, blue curve) and tension-reduced (N = 30, red curve, incubated with blebbistatin at 10 μM). Red and blue shaded regions indicate the respective standard error of the mean for each condition. Fluorescence intensities were normalized. Averaged curves were fitted with an exponential recovery (black dashed lines), with fit timescales of τ = 101.71 s (control) and 64.07 s (Bleb., 10 μM). (b) Recovery times from fits of individual recovery curves in control and blebbistatin-treated conditions corresponding to (a). Median values are 108.5 s (control) and 72.8 s (blebbistatin at 10 μM). (c) Estimated fraction of cortical α-actinin-4 in control and blebbistatin-treated conditions corresponding to (a). Median values of fit recovery timescales are 0.51 (control) and 0.46. (d) Cortical tensions in control and blebbistatin-treated conditions corresponding to measurements presented in (a). (e) Top: schematic of cellular response upon indirect photoactivation of myosin. Bottom: time series of confocal micrographs of the equatorial plane of the cell recorded during the photoactivation experiment. Fluorescence changes are typically too small to be assessed by eye. Scale bar, 10 μm. (f) Time evolution of the cortex/cytoplasm ratio = Ibound/Icyt of Actn4-mKate2 fluorescence (red curve) and corresponding time evolution of cortical tension (gray curve). The blue shaded region indicates the time interval during which cells were scanned with blue laser light (488 nm) to inactivate blebbistatin inside the cell. Averages were taken over 17 cells. Red and grey shaded regions indicate the standard errors of the mean of cortex/cytoplasm ratio and tension, respectively. (g) Averaged time evolution of cortex/cytoplasm ratio (red curve, as in f) with corresponding model fit (black dashed line). Fitting parameters are given in Table 1, fourth row. Curves were averaged over 17 cells. Before averaging, intensity curves were normalized to take the value one before photoinactivation (measurements are representative for at least two independent experiments. n.s., p > 0.05; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). To see this figure in color, go online.