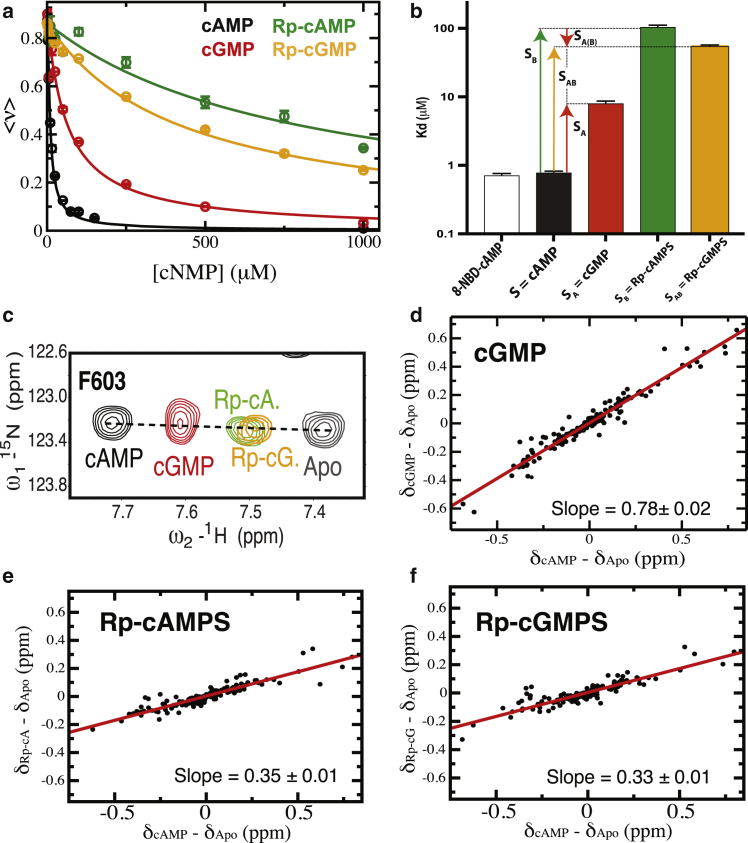
Figure 2.
Nonadditivity in the Rp/G double-ligand cycle for the HCN4 CNBD. (a) Binding competition isotherms for cNMP analogs versus the fluorescent 8-NBD-cAMP ligand. The cAMP analogs are those in the nonadditivity cycle of Fig. 1c. The Rp-cAMP/cGMP and Rp-cAMPS/cGMPS notations are used interchangeably in this paper. (b) Kd-values for the cAMP analogs in the previous panel, shown as a log scale bar plot. The Kd of 8-NBD-cAMP was measured by direct titration. The Kd-values are also reported in Table S1. Error bars were obtained from replicate measurements. The vertical arrows indicate the affinity changes caused by the A, B, A (B), and AB substitutions. The A (B) notation indicates that the A substitution occurs in the presence of B. (c) NH-HSQC spectral expansion of residue F603 in the HCN4 CNBD in the presence of saturating amounts of the cNMP analogs in the double-ligand cycle of Fig. 1c. The F603 position in the HSQC spectrum reports primarily on the shifts in the fast-exchanging HCN4 CNBD autoinhibitory P1 P2 equilibrium relative to the cAMP-bound and apo samples. (d–f) Chemical shift correlation plots for the cNMP analogs in (c). The slopes reflect the fractional activation relative to the cAMP-bound and apo HCN4 CNBD samples. Errors for the slopes were obtained from linear regressions. To see this figure in color, go online.