Figure 2.
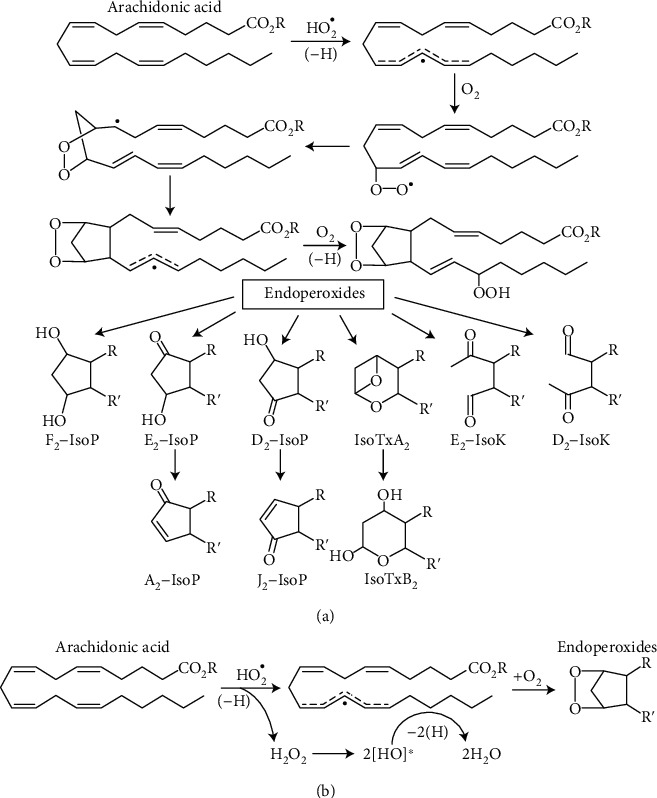
Autoxidation of arachidonic acid with rearrangements into different ring structures. (a) This part of Figure 2 (adapted from [81]) presents possible intermediate metabolites during autoxidation of arachidonic acid (AA) by some radical. HO2• is the only candidate to initiate autoxidation of AA that is esterified to a phospholipid [17]. (b) The proposed sequence of transformation of the HO2• and AA during IPLP [17]. Upon abstraction of the 1st H atom from AA, HO2• turns into H2O2, which in the hydrophobic environments undergoes homolytic decomposition into two molecules of •OH radical, which instantly abstract additional two H atoms from AA, producing 2H2O and the remnant of AA with complete disarranged double bonds. The extremely fast abstraction of three H atoms from any two double bonds creates a highly unstable molecule of AA, which very rapidly and randomly reacts with two molecules of O2 and undergoes intramolecular rearrangements, which results in a large number of positional isomers and stereoisomers. The more PUFA has double bonds, the larger is the number of positional isomers and stereoisomers. Abbreviations: F2-IsoP, E2-IsoP, D2-IsoP are Isoprostanes with rings, correspondingly F2, E2, D2, or A2 and J2; IsoTxA2 and IsoTxB2 are isothromboxanes with rings A2 and B2, correspondingly, formed from Prostanglandin-H2 (PGH2); E2-IsoK and D2-IsoK are Isoketals with rings E2 and D2.
