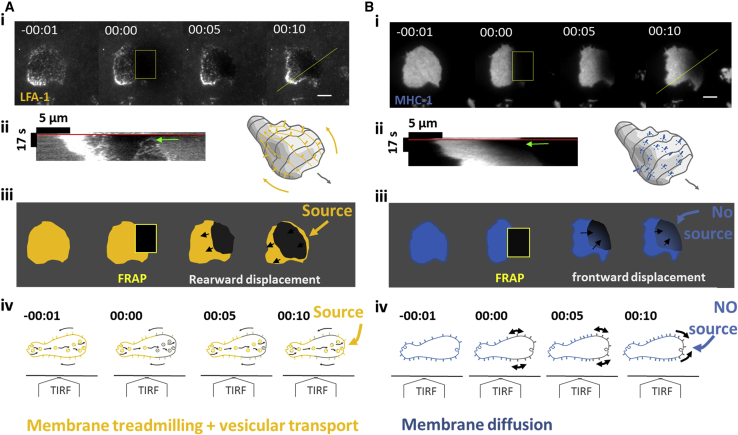
Figure 8.
Treadmilling actin-bound transmembrane protein LFA-1 is recycled by internal vesicular transport, whereas diffusive transmembrane protein MHC-1 is not. (A) Staining of high affinity LFA-1 and (B) of MHC-1. (i) Sequences of TIRF images before FRAP, after FRAP of cell leading edge, and then 5 and 10 s after FRAP. Scale bars, 5 μm. See also Video S16. (ii) Kymographs along yellow lines of figures (A and B), with red lines indicating FRAP time and green arrows pointing at the cell front shortly after FRAP. 3D cartoon illustrates backward treadmilling of LFA-1 and 2D diffusion of MHC-1. (iii) Schematics of experimental results in (i) illustrate that fluorescence recovers from the cell leading edge for LFA-1, revealing a source at cell front, and from the back for MHC-1, revealing the absence of source at cell front. (iv) Side view schematics of nonadherent cells observed with a TIRF objective illustrate the dynamics of transmembrane proteins evidenced in TIRF experiments. For actin-binding LFA-1, the source at cell front reveals internal frontward transport of fresh material exocytosed at cell front, whereas for nonactin-binding MHC-1, purely diffusive transport dominates. To see this figure in color, go online.