Abstract
PURPOSE
To describe the incidence, relative risk, and risk factors for chronic comorbidities in survivors of adolescent and young adult (AYA) cancer.
METHODS
This retrospective cohort study included 2-year survivors of AYA cancer diagnosed between age 15 and 39 years at Kaiser Permanente Southern California from 2000 to 2012. A comparison cohort without cancer was individually matched (13:1) to survivors of cancer on age, sex, and calendar year. Using electronic medical records, all participants were followed through December 31, 2014, for chronic comorbidity diagnoses. Poisson regression was used to evaluate the association between cancer survivor status and risk of developing each comorbidity. The associations between cumulative exposure to chemotherapy and radiation therapy and selected comorbidities were examined for survivors of cancer.
RESULTS
The cohort included 6,778 survivors of AYA cancer and 87,737 persons without a history of cancer. The incidence rate ratio (IRR) for survivors of cancer was significantly increased for nearly all comorbidities examined. IRR ranged from 1.3 (95% CI, 1.2 to 1.4) for dyslipidemia to 8.3 (95% CI, 4.6 to 14.9) for avascular necrosis. Survivors of AYA cancer had a 2- to 3-fold increased risk for cardiomyopathy, stroke, premature ovarian failure, chronic liver disease, and renal failure. Among survivors of cancer, significant associations between chemotherapy and radiation therapy exposures and late effects of cardiomyopathy, hearing loss, stroke, thyroid disorders, and diabetes were observed from the multivariable analyses. Forty percent of survivors of AYA cancer had multiple (≥ 2) comorbidities at 10 years after index date, compared with 20% of those without cancer.
CONCLUSION
Risk of developing comorbidities is increased in survivors of AYA cancer compared with the general population. Specific cancer treatment exposures were associated with risk of developing different comorbidities. These findings have important implications for survivorship care planning and patient education.
INTRODUCTION
The incidence of cancer in adolescents and young adults (AYAs) has been increasing over the past 25 years.1,2 With an annual incidence of > 70,000 cases3 and 5-year survival exceeding 80%,4,5 the number of survivors of AYA cancer living in the United States is estimated to be > 633,000 and will continue to grow.6 Like survivors of childhood cancer, survivors of AYA cancer are at risk for late effects of cancer treatments; the detrimental impact of these late effects on years and quality of life lost can be substantial.7-10
Context
Key Objective
The number of survivors of adolescent and young adult (AYA) cancer, defined as cancer diagnosed in a person between age 15 and 39 years, is estimated to be > 633,000 in the United States and will continue to grow. Although extensive research has described the risk of chronic comorbidities among survivors of childhood cancer, such data on survivors of AYA cancer remain sparse. This study characterized the incidence, relative risk, and risk factors for chronic comorbidities in survivors of AYA cancer.
Knowledge Generated
Survivors of AYA cancer have increased risk of developing a wide array of chronic comorbidities compared with matched individuals without a history of cancer. Specific treatment exposures, including chemotherapy and radiation therapy, are associated with increased risk of late effects in multiple organ systems among survivors of AYA cancer.
Relevance
Data from this study may help provide much-needed information for the development of personalized survivorship care plans for survivors of AYA cancer.
Although extensive research has described the risk of chronic comorbidities among survivors of childhood cancer,11-13 these data may not be applicable to survivors of cancers diagnosed later in life. The risk and risk factors for comorbidities may vary across age groups as a result of differences in primary cancers, treatment exposures, age at exposures, and background incidence.14 There is a paucity of longitudinal data on the absolute and relative magnitude of risk for chronic health problems in survivors of AYA cancer. Moreover, there have been no studies to describe the specific risks to subgroups of survivors defined by demographic characteristics and cancer treatment exposures. This lack of high-quality data for AYAs with cancer has impeded the development of evidence-based early screening recommendations and interventions to limit the adverse impact of cancer and its treatment in these survivors.
To address these gaps in the literature, we evaluated the development of chronic comorbidities in survivors of AYA cancer who were members of Kaiser Permanente Southern California (KPSC) using a matched cohort design. We used an internal noncancer comparison cohort with similar demographics, socioeconomic status, and access to health care and took advantage of detailed treatment and diagnosis data from electronic medical records.
METHODS
Study Setting and Study Population
KPSC is an integrated health care organization that provides comprehensive health services to > 4.4 million racially, ethnically, and socioeconomically diverse members who are broadly representative of residents in southern California.15 KPSC has high membership retention; we have shown that among AYAs diagnosed with cancer, 77% and 62% remain KPSC members 5 and 10 years after diagnosis, respectively.16
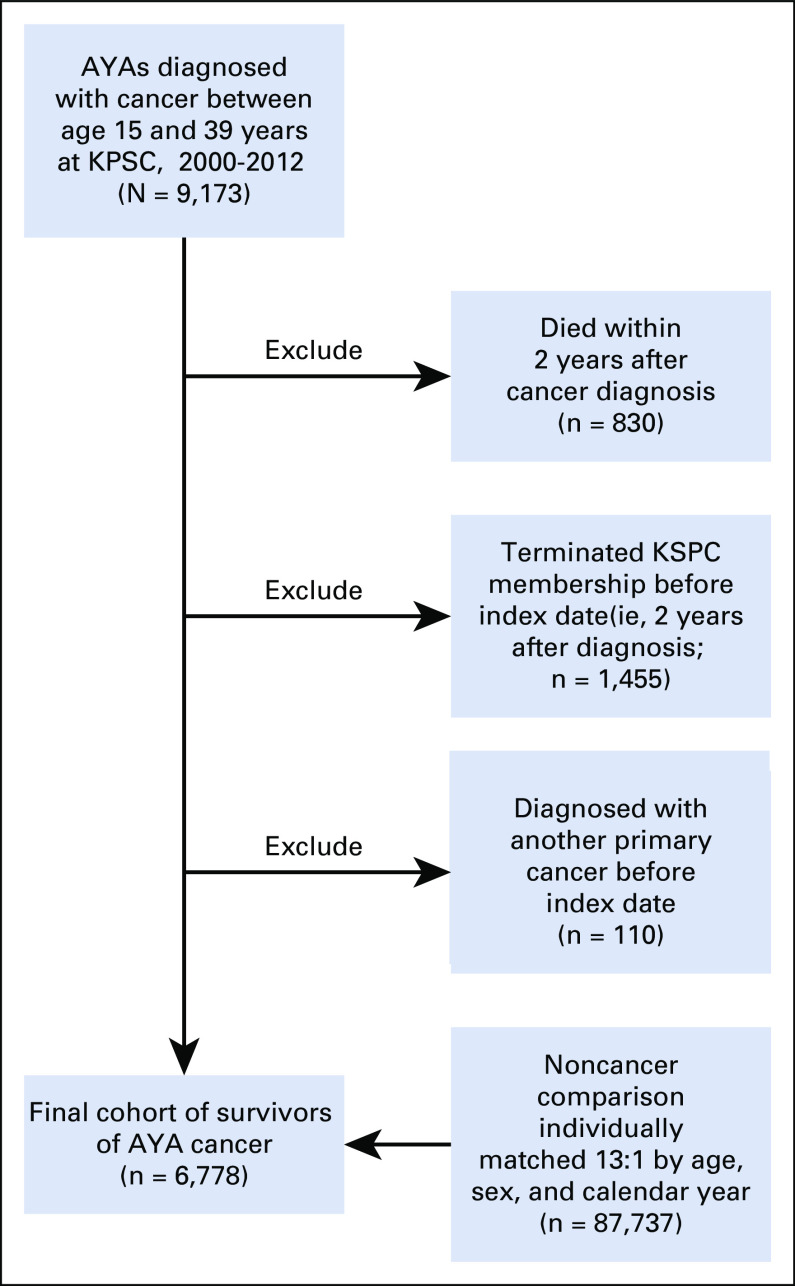
We identified survivors of AYA cancer using KPSC’s SEER-affiliated cancer registry. KPSC members who met the following criteria were included in the cohort of survivors of AYA cancer: diagnosed with invasive cancer at age 15-39 years between 2000 and 2012 at KPSC; survived ≥ 2 years (index date) after cancer diagnosis (being cancer free at 2 years was not required); and retained KPSC membership at index date. We used 2-year survival as the cutoff for study inclusion because our goal was to characterize health outcomes after the completion of active treatment, and the majority (> 90%) of our AYA cancer cohort completed treatment within 2 years. In addition, the 2-year time point represented a junction whereby survivors are most likely transitioned from active therapy to long-term follow-up programs or to their primary care providers. Survivors diagnosed with another primary cancer before the index date were excluded.
KPSC members without a history of cancer were included as a reference group and individually matched 13:1 to survivors of AYA cancer by age (yearly), sex, and calendar year (age of the noncancer participants had to match the cancer survivor’s age in the same calendar year of the cancer survivor’s index date). Each noncancer participant was subsequently assigned an index date that was the index date of the survivor of cancer to which he or she was matched. Individuals in the noncancer comparison cohort were identified from KPSC members in the year of the corresponding cancer diagnosis for a survivor of cancer and remained a KPSC member 2 years thereafter (ie, at index date or later). Additional record linkage with the California Cancer Registry was performed to exclude the comparison participants who had a history of cancer before their KPSC enrollment. This study was approved and the requirement of informed consent was waived by KPSC’s Institutional Review Board.
Data Collection
All study participants were followed from index date to KPSC disenrollment, death, or end of 2014, whichever came first. Comorbidities that are known late effects of cancer treatments or common in this age group were included for examination. The presence of these comorbidities before or after the index date was captured using previously validated or published approaches whenever possible17-22 (Data Supplement). Covariates of interest included demographics, cancer characteristics, chemotherapy exposures (agent, cumulative dose), radiation therapy (anatomic site, cumulative dose), and death information. All covariate data were collected using KPSC’s electronic health records and cancer registries, except for information on death, which was supplemented by outside claims, California State death files, and national Social Security death files.
Statistical Analysis
The distribution of demographic, cancer, and treatment characteristics and the incidence rate of each comorbidity were calculated. For each comorbidity, those with a diagnosis date that preceded the index date (prevalent cases) were excluded from the calculation of incidence rate and incidence rate ratios (IRRs) because we were primarily interested in late effects of treatment to inform screening guidelines after cancer treatment. The crude and adjusted IRRs of each comorbidity associated with being a survivor of AYA cancer compared with those in the noncancer cohort were estimated using bivariable and multivariable Poisson regression adjusting for age, sex, and race and ethnicity. The Bayesian Improved Surname Geocoding imputation method was used to impute for the missing (approximately 13%) race and ethnicity information in the noncancer comparison participants.23 The cumulative incidence of each comorbidity and of ≥ 2 comorbidities was calculated using nonparametric methods accounting for competing risk.24 Differences in cumulative incidence were tested using the Gray’s test.25 The trend for prevalence of ≥ 2 comorbidities over time was also plotted.
Among survivors of AYA cancer, Poisson regression was performed to evaluate the association between treatment and risk of selected comorbidities with a known or suspected association with these exposures. Because the vast majority of survivors (> 90%) received all their cancer treatment within 2 years after diagnosis, treatment exposure was considered a time-fixed variable representing the cumulative exposure before the index date. A potential dose-response relationship was evaluated in crude Poisson regression; appropriate cutoff for the cumulative dose of a chemotherapy agent was determined based on its dose distribution (ie, tertile). A similar analytical strategy was used to evaluate the relationship for radiation therapy.
Multivariable Poisson regressions were performed to evaluate the effects of cancer treatment on common health outcomes, adjusting for age, sex, and race and ethnicity. When applicable, the joint effect of chemotherapy and radiation therapy was evaluated by creating mutually exclusive exposure categories of the combined exposure (eg, anthracycline and chest radiation for cardiomyopathy, platinum agents and head radiation for hearing loss). Sensitivity analyses were conducted to eliminate potential confounding by the initial diagnosis; for premature ovarian failure, avascular necrosis, and thyroid disorders, the sensitivity analyses excluded survivors of ovarian cancer, bone cancer, and thyroid cancer, respectively. All analyses were carried out using SAS Version 9.3 (SAS Institute, Cary, NC).
RESULTS
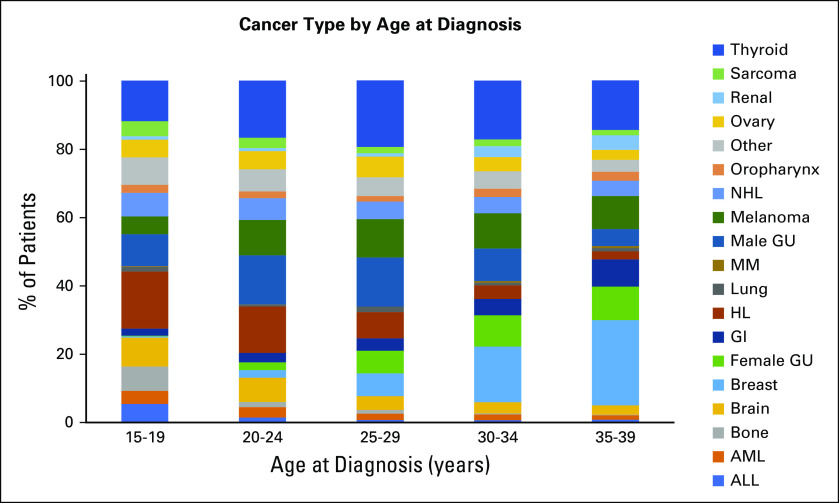
A total of 9,173 KPSC members were diagnosed with an invasive cancer between 15 and 39 years of age from 2000 to 2012. Of these, 6,778 survivors met the inclusion criteria and were matched to 87,737 individuals without cancer (Appendix Fig A1, online only). Among survivors of AYA cancer, median age at initial cancer diagnosis was 31 years (15-19 years, 8%; 20-29 years, 25%; and 30-39 years, 67%). Approximately 65% of survivors were female, and 42% were non-Hispanic White. The most common cancer diagnoses were breast cancer (16%) and thyroid cancer (16%), followed by lymphoma (11%) and melanoma (10%). There was substantial heterogeneity of cancer type distribution by 5-year age groups (Appendix Fig A2, online only). Approximately a quarter of survivors of AYA cancer (23%) received external-beam radiation therapy. Exposure to selected chemotherapy agents ranged from 3% (epipodophyllotoxins) to 24% (anthracyclines; Table 1). The median follow-up time after cancer diagnosis was 5.1 years.
TABLE 1.
Demographic and Clinical Characteristics of the Study Population
During the study follow-up, a total of 1,443 survivors of cancer (17%) developed ≥ 1 comorbidity, with an incidence rate of 66 per 1,000 person-years. The adjusted IRR of developing any comorbidity among survivors of cancer compared with the noncancer cohort was 1.47 (95% CI, 1.39 to 1.55; Table 2). The most common comorbidities among survivors of cancer were dyslipidemia (22 per 1,000 person-years), hypertension (16 per 1,000 person-years), diabetes (10 per 1,000 person-years), thyroid disorders (9 per 1000 person-years), and severe depression or anxiety (8 per 1,000 person-years; Table 2). With the exception of vision loss and asthma, survivors of cancer were significantly more likely to develop a comorbidity compared with the noncancer cohort. The IRR was highest for avascular necrosis (IRR, 8.25; 95% CI, 4.58 to 14.85), followed by osteoporosis (IRR, 5.75; 95% CI, 3.71 to 8.93), joint replacement (IRR, 3.89; 95% CI, 2.43 to 6.22), stroke (IRR, 3.19; 95% CI, 2.37 to 4.29), premature ovarian failure (IRR, 2.87; 95% CI, 1.56 to 5.28), and cardiomyopathy or heart failure (IRR, 2.64; 95% CI, 1.84 to 3.79; Table 2). The adjusted IRR of developing ≥ 2 incident comorbidities was 1.63 (95% CI, 1.48 to 1.78).
TABLE 2.
IR and IRR of Comorbidities Comparing Survivors of Adolescent and Young Adult Cancer to Matched Noncancer Comparison Cohort
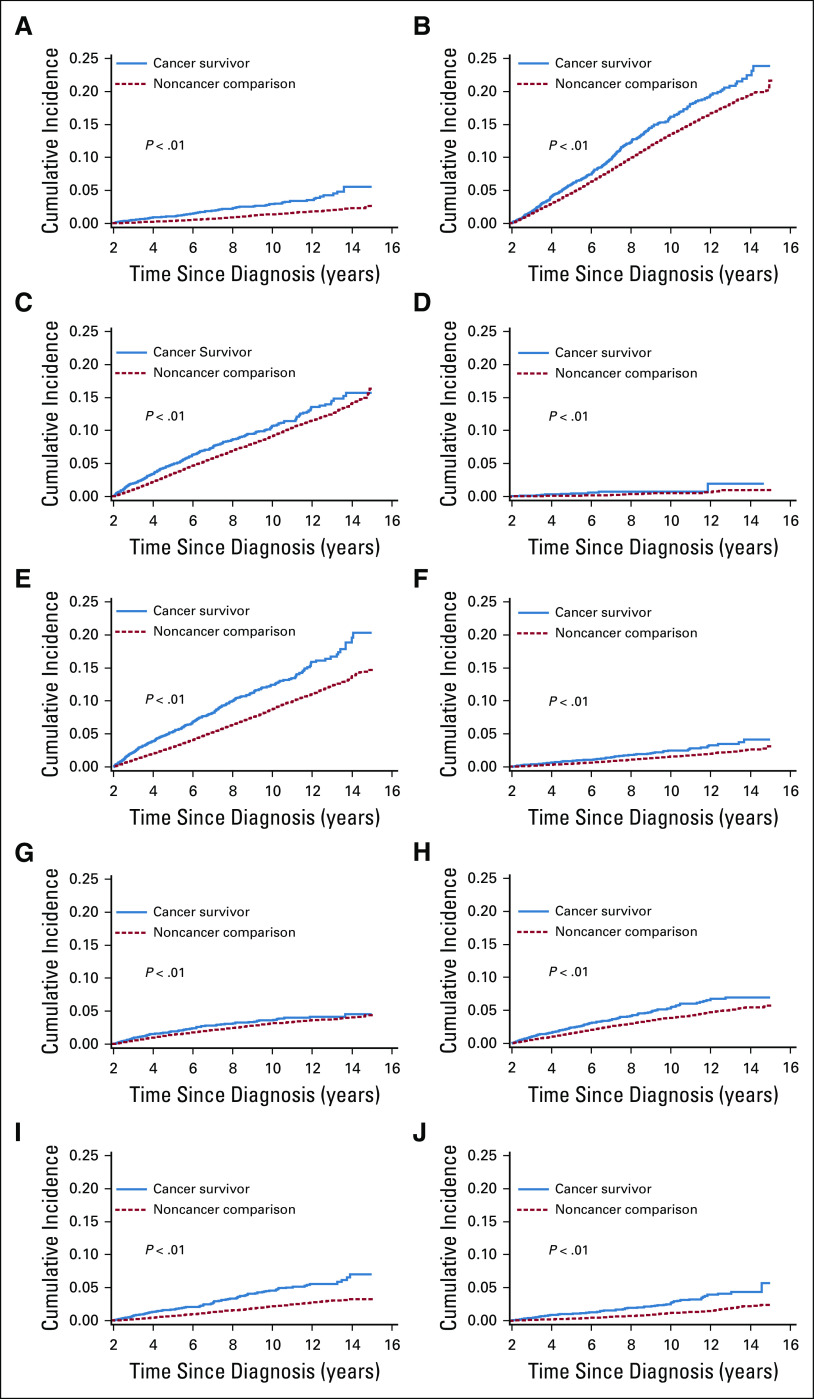
The cumulative incidence curves for any comorbidity and for comorbidities by organ system are shown in Figure 1 and Appendix Figure A3 (online only). Fig 1 also shows the prevalence of ≥ 2 comorbidities over time. For survivors of AYA cancer, the prevalence of multiple comorbidities approached 40% at 10 years after the index date, compared with 20% for those without cancer (P < .001). The combinations of the first 2 incident comorbidities among survivors of cancer are shown in the Data Supplement.
FIG 1.
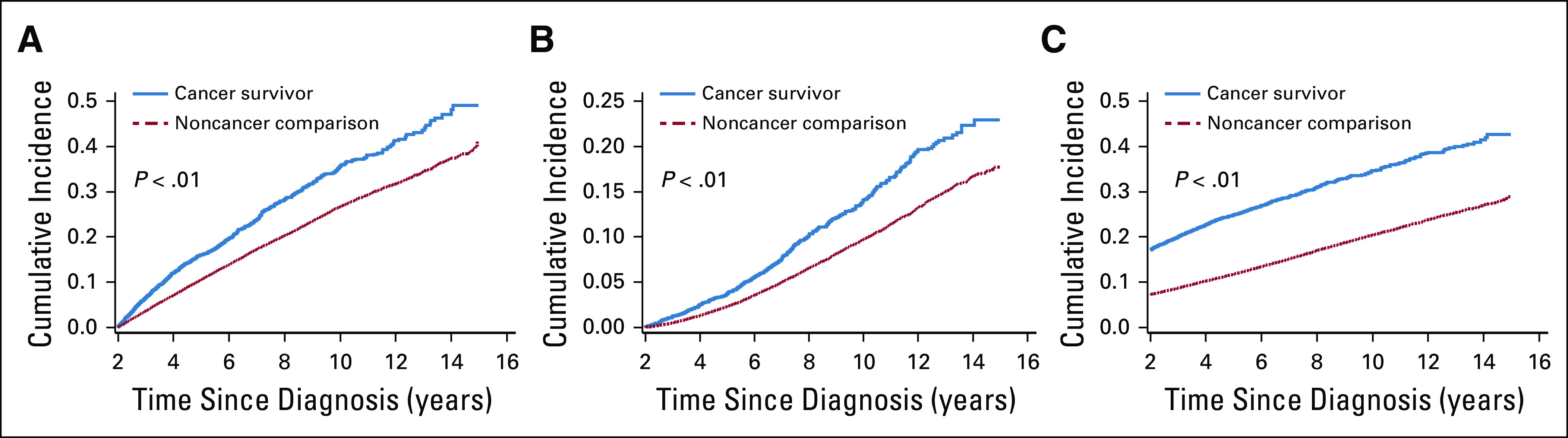
Incidence of (A) any comorbidity, (B) ≥ 2 new comorbidities, and (C) ≥ 2 comorbidities by cancer survivor status.
Within Survivors of AYA Cancer
When we examined the crude association between exposure to selected chemotherapy agents and the development of specific comorbidities (Table 3), use of alkylating agents (IRR, 2.8 [95% CI, 1.2 to 6.2] for > 2,500 mg/m2), anthracyclines (IRR, 3.3 [95% CI, 1.6 to 6.9] for > 240 mg/m2), and trastuzumab (IRR, 9.1 [95% CI, 3.2 to 25.6] for > 4,000 mg/m2) was significantly associated with risk of cardiomyopathy or heart failure. Use of alkylating agents (IRR, 4.5 [95% CI, 1.4 to 15.0] for > 2,500 mg/m2) and platinum agents (IRR, 9.0 [95% CI, 2.8 to 29.2] for > 450 mg/m2) was significantly associated with premature ovarian failure. Use of methotrexate (IRR, 21.6 [95% CI, 8.8 to 52.8] for any dose) and corticosteroids (IRR, 5.4 [95% CI, 1.8 to 16.0] for any dose) was significantly associated with avascular necrosis. Use of bleomycin was significantly associated with pulmonary fibrosis (IRR, 4.7 [95% CI, 1.0 to 21.7] for > 120 mg/m2), and use of corticosteroids was significantly associated with osteoporosis (IRR, 2.3 [95% CI, 1.1 to 4.9] for any dose).
TABLE 3.
Crude Associations Between Chemotherapy Exposure and Selected Comorbidity Outcomes
Table 4 lists the crude IRR estimates for selected comorbidities associated with radiation exposure (compared with no exposure). Radiation therapy to the head and neck area was significantly associated with risk of stroke (IRR, 3.1 [95% CI, 1.6 to 6.0] for ≥ 30 Gy), hearing loss (IRR, 3.0 [95% CI, 1.6 to 5.6] for ≥ 30 Gy [head only]), vision loss (IRR, 8.1 [95% CI, 2.7 to 24.2] for ≥ 30 Gy [head only]), thyroid disorders (IRR, 3.4 [95% CI, 2.4 to 4.7] for ≥ 30 Gy), and diabetes (IRR, 1.9 [95% CI, 1.3 to 2.8] for ≥ 30 Gy [head only]). Chest radiation therapy was associated with risk of cardiomyopathy or heart failure (IRR, 2.6 [95% CI, 1.2 to 5.7] for ≥ 30 Gy).
TABLE 4.
Crude Associations Between Radiation Exposure and Selected Comorbidity Outcomes
In multivariable analyses, the following agents were significantly and independently associated with risk of cardiomyopathy: anthracycline > 240 mg/m2 (IRR, 3.3; 95% CI, 1.0 to 10.67) and trastuzumab > 4,000 mg/m2 (IRR, 8.1; 95% CI, 2.5 to 25.5; Table 5). A combined exposure to platinum (> 450 mg/m2) and radiation to the head (≥ 30 Gy) was associated with increased risk of hearing loss (IRR, 14.9; 95% CI, 5.9 to 37.2). Radiation therapy to the head and neck (≥ 30 Gy) was associated with risk of stroke (IRR, 3.5; 95% CI, 1.8 to 6.8). For thyroid disorders, significant risk factors included female sex and head and neck radiation (≥ 30 Gy; IRR, 3.1; 95% CI, 2.2 to 4.4). For diabetes, significant risk factors included older age at diagnosis (30-39 years); non-Hispanic Black, Hispanic, and Asian race/ethnicity; and head radiation (≥ 30 Gy; IRR, 1.9; 95% CI, 1.3 to 2.9). Similar results were obtained in sensitivity analyses that excluded survivors of ovarian cancer (premature ovarian failure), bone cancer (avascular necrosis), and thyroid cancer (thyroid disorders).
TABLE 5.
Risk Factors for Comorbidity Outcomes Among Survivors of Adolescent and Young Adult Cancer in Adjusted Poisson Regression
DISCUSSION
In this study, we describe the incidence and relative risk of development of chronic comorbidities among survivors of AYA cancer compared with controls without a history of cancer. We found that the risk of nearly all comorbidities was significantly elevated in survivors of cancer, with magnitudes of IRR ranging from 1.3 for dyslipidemia to 8.3 for avascular necrosis. Moreover, 2 in 5 survivors had multiple comorbidities just 10 years after the index date. Among survivors of AYA cancer, we observed significant associations between treatment exposures and certain comorbidities such as cardiomyopathy, hearing loss, stroke, thyroid disorders, and diabetes. These data highlight the burden of adverse long-term health outcomes of cancer and its treatment in survivors of AYA cancer and will facilitate the identification of high-risk survivors who may benefit from tailored surveillance and prevention strategies.
Long-term survival outcomes in AYAs with cancer has not improved to the extent seen in children with cancer.5 Lack of data on long-term health outcomes and treatment late effects may be key knowledge gaps for improving outcomes in these patients. To our knowledge, this is the first study to systematically characterize the development of chronic comorbidities among survivors of AYA cancer in the United States and to examine the relationship between these comorbidities and treatment exposures in this unique group. A few US-based studies have reported on the comorbidity burden among survivors of AYA cancer, although most are cross-sectional and subject to recall and participation bias as a result of the use of self-reported questionnaires and/or lack of a comparison group.26-29 Longitudinal studies have described hospitalizations for survivors of AYA cancer in Denmark,30-33 but these studies have not included more common health conditions that can be diagnosed and managed on an outpatient basis. As such, the current study represents an important and more comprehensive assessment of the long-term burden of comorbidities in survivors of AYA cancers.
Compared with survivors of childhood cancer, the relative risk increase for survivors of AYA cancer appears to be smaller in magnitude across most comorbidities. Data from the Childhood Cancer Survivors Study (CCSS) showed that for grade 3 or 4 cardiac events, the IRR was 7.5 for survivors of childhood cancer. For grade 3 or 4 pulmonary disease, renal disease, disorders of hearing, speech, or vision, and musculoskeletal conditions, the IRRs were 3.1, 8.1, 5.8, and 77.1, respectively.11 In comparison, in the current study, the IRRs for severe cardiovascular diseases, severe pulmonary diseases, renal failure, hearing loss, vision loss, and musculoskeletal conditions were 2.1, 2.3, 2.3, 1.7, 1.5, and 2.6, respectively. Although the definitions of comorbidities, study design, and follow-up time between our study and the CCSS are different, the differences may still reflect the intensity of cancer treatments, the effect of these treatments on developing organs versus mature organs, and background incidence in the general population. Importantly, these differences again highlight the need for age-appropriate survivorship guidelines for survivors of AYA cancer.
The examination of the relationship between treatment exposure and selected health outcomes was based on a priori knowledge about treatment-related late effects in survivors of childhood cancer,34 as well as those reported in survivors of adult-onset cancers (eg, trastuzumab). Although we confirmed previously reported associations between anthracyclines and trastuzumab and cardiomyopathy, platinums and head and neck radiation and hearing loss, head and neck radiation and stroke, head and neck radiation and thyroid diseases, and head and neck radiation and diabetes, there were other instances where the association could not be confirmed. These included, for example, the association between platinum exposure and myocardial infarction, methotrexate and renal failure, and pelvic radiation and premature ovarian failure. There are several possible explanations for these null results, including a true lack of association, limited power for some analyses, or the need for longer follow-up for certain late effects (eg, myocardial infarction). Several other risk factors based on patient characteristics were also found, which vary for different comorbidities.
National guidelines specific for AYA oncology were not available until 2016.35 For the survivorship care recommendations, the National Comprehensive Cancer Network (NCCN) AYA guidelines generally follow the Children’s Oncology Group long-term follow-up guidelines, noting that these guidelines do not apply to survivors of common adult cancer types (eg, breast and colorectal cancer). There also remains room for clinical judgement for the survivorship screening tests recommend by the NCCN AYA oncology guidelines. For example, the recommendations for cardiomyopathy screening with echocardiography were for 2- to 5-year intervals for a range of anthracycline and chest radiation exposures, but the specific intervals of screening based on level of treatment exposure were not specified. A study by Barthel et al36 found that, for AYA cancers, age-related recommendations from several survivorship care guidelines disagree on the link between treatment exposures and late effects, who should be screened, the screening intervals, and the screening test to be used.35 Unique challenges for survivorship care for AYA cancer may stem from the heterogeneity of cancer type distributions and treatment variations for the same cancer (eg, pediatric v adult oncology) within the AYA age range. There is certainly a need for additional research to further inform guideline development and strategies to promote the adoption of these guidelines to help improve long-term outcomes for these survivors of cancer.
There are several limitations of this study that should be considered. First, for comorbidities that require medical management but are mostly asymptomatic (eg, dyslipidemia, hypertension, osteoporosis), there may be differential surveillance patterns; survivors of cancer likely have more frequent health care encounters and/or are more likely to be screened and diagnosed with these conditions. As a result, the findings for these comorbidities should be interpreted with caution. Second, the number of events for some comorbidities were small, which limited our ability to perform multivariable analyses for their risk factors. Third, our follow-up was limited to 14 years after diagnosis, with a median follow-up of 5 years after diagnosis. As a result, this study may not be representative of late-occurring (> 10-15 years) comorbidities in survivors of AYA cancer. Future studies should include longer follow-up to further inform the long-term trajectory of health risk. Finally, we did not have comprehensive data on lifestyle factors such as smoking, alcohol use, and exercise to evaluate whether they may have confounded the observed associations.
Despite the limitations, our study has several important strengths. We have longitudinal data on the development of health outcomes and a carefully matched internal comparison cohort, providing us with robust estimates for the IRR of health outcomes. We also included cancer treatment in our analyses, allowing us to examine the association between select exposures and more common comorbidities. Our racially and ethnically diverse survivor population made our findings uniquely relevant for populations in the United States compared with studies from Scandinavian countries. In addition, the relatively equal access to health care in our membership minimized the likelihood of confounding as a result of insurance coverage; previous studies have highlighted the challenges of conducting studies in survivors of AYA cancers as a result of inequities in access to care.37 Leveraging the stable membership of KPSC, we were able to study the health outcomes of AYAs long after their cancer diagnosis, allowing us to address important gaps in the literature.
In conclusion, this study provided a comprehensive overview of the incidence and relative risk of chronic comorbidities in long-term survivors of AYA cancer compared with matched individuals without a history of cancer. We showed that the risk of developing chronic comorbidities was increased across nearly all outcomes examined and highlighted important treatment-related modifiers of risk in these survivors. These data may help provide much-needed information for the development of personalized survivorship care plans for survivors of AYA cancer, taking into consideration the unique phenotypes of comorbidities over time. Importantly, it may set the stage for prevention, early diagnosis, or intervention strategies to mitigate long-term comorbidity burden in this growing population of long-term survivors.
ACKNOWLEDGMENT
We thank the patients of Kaiser Permanente for helping us improve care through the use of information collected through our electronic health record systems.
APPENDIX
FIG A1.
Study population flowchart. AYA, adolescent and young adult; KPSC, Kaiser Permanente Southern California.
FIG A2.
Distribution of cancer types by 5-year age groups within survivors of adolescent and young adult cancer. Cancer type shown in the same order (from top to bottom) in every age group as in the right text panel. ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; GU, genitourinary; HL, Hodgkin lymphoma; MM, multiple myeloma; NHL, non-Hodgkin lymphoma.
FIG A3.
Incidence of condition by cancer survivor status: (A) cardiovascular, (B) dyslipidemia, (C) hypertension, (D) premature ovarian failure, (E) other endocrine disease, (F) neurosensory, (G) pulmonary, (H) severe depression or anxiety, (I) hepatic or renal disease, and (J) musculoskeletal.
PRIOR PRESENTATION
Presented in part at the 54th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2018.
SUPPORT
Supported by Grant No. RSG-15-016-01-CPHPS (C.C.) from the American Cancer Society.
AUTHOR CONTRIBUTIONS
Conception and design: Chun Chao, Smita Bhatia, Po-Yin Samuel Huang, Robert Cooper, Saro H. Armenian
Administrative support: Kimberly L. Cannavale
Collection and assembly of data: Chun Chao, Lanfang Xu, Po-Yin Samuel Huang
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Chronic Comorbidities Among Survivors of Adolescent and Young Adult Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Chun Chao
Employment: Kaiser Permanente
Stock and Other Ownership Interests: Merck (I), United Health Care (I)
Research Funding: Merck (Inst), Seattle Genetics (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Bleyer A, O’Leary M, Barr R, et al: Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: 1975-2000. http://seer.cancer.gov/publications/aya/
- 2.Burkhamer J, Kriebel D, Clapp R. The increasing toll of adolescent cancer incidence in the US. PLoS One. 2017;12:e0172986. doi: 10.1371/journal.pone.0172986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hellmann K. Dexrazoxane-associated risk for secondary malignancies in pediatric Hodgkin’s disease: A claim without evidence. J Clin Oncol. 2007;25:4689–4691. doi: 10.1200/JCO.2007.12.6888. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Moke DJ, Tsai KY, et al. A reappraisal of sex-specific cancer survival trends among adolescents and young adults in the United States. J Natl Cancer Inst. 2019;111:509–518. doi: 10.1093/jnci/djy140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keegan TH, Ries LA, Barr RD, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016;122:1009–1016. doi: 10.1002/cncr.29869. [DOI] [PubMed] [Google Scholar]
- 6.Berdal G, Halvorsen S, van der Heijde D, et al. Restrictive pulmonary function is more prevalent in patients with ankylosing spondylitis than in matched population controls and is associated with impaired spinal mobility: A comparative study. Arthritis Res Ther. 2012;14:R19. doi: 10.1186/ar3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson P, McDonald FE, Zebrack B, et al. Emerging issues among adolescent and young adult cancer survivors. Semin Oncol Nurs. 2015;31:53–59. doi: 10.1016/j.soncn.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Armenian SH, Xu L, Ky B, et al. Cardiovascular disease among survivors of adult-onset cancer: A community-based retrospective cohort study. J Clin Oncol. 2016;34:1122–1130. doi: 10.1200/JCO.2015.64.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Belt-Dusebout AW, Nuver J, de Wit R, et al. Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2006;24:467–475. doi: 10.1200/JCO.2005.02.7193. [DOI] [PubMed] [Google Scholar]
- 10.Fosså SD, Aass N, Harvei S, et al. Increased mortality rates in young and middle-aged patients with malignant germ cell tumours. Br J Cancer. 2004;90:607–612. doi: 10.1038/sj.bjc.6601558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: A review of published findings. J Clin Oncol. 2009;27:2339–2355. doi: 10.1200/JCO.2008.21.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hudson MM, Mulrooney DA, Bowers DC, et al. High-risk populations identified in Childhood Cancer Survivor Study investigations: Implications for risk-based surveillance. J Clin Oncol. 2009;27:2405–2414. doi: 10.1200/JCO.2008.21.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 14.Woodward E, Jessop M, Glaser A, et al. Late effects in survivors of teenage and young adult cancer: Does age matter? Ann Oncol. 2011;22:2561–2568. doi: 10.1093/annonc/mdr044. [DOI] [PubMed] [Google Scholar]
- 15.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: Comparison with US Census Bureau data. Perm J. 2012;16:37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao C, Chiu V, Mueller LA, et al. Exploring the feasibility of establishing a retrospective cohort of survivors of adolescent and young adult cancer to study long-term health outcomes in an integrated managed care environment. J Adolesc Young Adult Oncol. 2013;2:59–65. doi: 10.1089/jayao.2012.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armenian SH, Xu L, Cannavale KL, et al. Cause-specific mortality in survivors of adolescent and young adult cancer. Cancer. 2020;126:2305–2316. doi: 10.1002/cncr.32775. [DOI] [PubMed] [Google Scholar]
- 18.Chao C, Xu L, Bhatia S, et al. Cardiovascular disease risk profiles in survivors of adolescent and young adult (AYA) cancer: The Kaiser Permanente AYA Cancer Survivors Study. J Clin Oncol. 2016;34:1626–1633. doi: 10.1200/JCO.2015.65.5845. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence JM, Black MH, Zhang JL, et al. Validation of pediatric diabetes case identification approaches for diagnosed cases by using information in the electronic health records of a large integrated managed health care organization. Am J Epidemiol. 2014;179:27–38. doi: 10.1093/aje/kwt230. [DOI] [PubMed] [Google Scholar]
- 20.Derose SF, Rutkowski MP, Crooks PW, et al. Racial differences in estimated GFR decline, ESRD, and mortality in an integrated health system. Am J Kidney Dis. 2013;62:236–244. doi: 10.1053/j.ajkd.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher D, Coleman KJ, Arterburn DE, et al. Mental illness in bariatric surgery: A cohort study from the PORTAL network. Obesity (Silver Spring) 2017;25:850–856. doi: 10.1002/oby.21814. [DOI] [PubMed] [Google Scholar]
- 22.Ray WA, Griffin MR, Fought RL, et al. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45:703–714. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]
- 23.Derose SF, Contreras R, Coleman KJ, et al. Race and ethnicity data quality and imputation using U.S. Census data in an integrated health system: The Kaiser Permanente Southern California experience. Med Care Res Rev. 2013;70:330–345. doi: 10.1177/1077558712466293. [DOI] [PubMed] [Google Scholar]
- 24. Lin G, So Y, Johnston G: Analyzing survival data with competing risks using SAS software. https://support.sas.com/resources/papers/proceedings12/344-2012.pdf.
- 25.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 26.Tai E, Buchanan N, Townsend J, et al. Health status of adolescent and young adult cancer survivors. Cancer. 2012;118:4884–4891. doi: 10.1002/cncr.27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaul S, Veeranki SP, Rodriguez AM, et al. Cigarette smoking, comorbidity, and general health among survivors of adolescent and young adult cancer. Cancer. 2016;122:2895–2905. doi: 10.1002/cncr.30086. [DOI] [PubMed] [Google Scholar]
- 28.Wu XC, Prasad PK, Landry I, et al. Impact of the AYA HOPE comorbidity index on assessing health care service needs and health status among adolescents and young adults with cancer. Cancer Epidemiol Biomarkers Prev. 2015;24:1844–1849. doi: 10.1158/1055-9965.EPI-15-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smitherman AB, Anderson C, Lund JL, et al. Frailty and comorbidities among survivors of adolescent and young adult cancer: A cross-sectional examination of a hospital-based survivorship cohort. J Adolesc Young Adult Oncol. 2018;7:374–383. doi: 10.1089/jayao.2017.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen MV, Rugbjerg K, de Fine Licht S, et al. Endocrine late effects in survivors of cancer in adolescence and young adulthood: A Danish population-based cohort study. JAMA Netw Open. 2018;1:e180349. doi: 10.1001/jamanetworkopen.2018.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rugbjerg K, Mellemkjaer L, Boice JD, et al. Cardiovascular disease in survivors of adolescent and young adult cancer: A Danish cohort study, 1943-2009. J Natl Cancer Inst. 2014;106:dju110. doi: 10.1093/jnci/dju110. [DOI] [PubMed] [Google Scholar]
- 32.Rugbjerg K, Olsen JH. Long-term risk of hospitalization for somatic diseases in survivors of adolescent or young adult cancer. JAMA Oncol. 2016;2:193–200. doi: 10.1001/jamaoncol.2015.4393. [DOI] [PubMed] [Google Scholar]
- 33.de Fine Licht S, Maraldo MV, Specht L, et al. Risk factors for cardiovascular disease in 5-year survivors of adolescent and young adult cancer: A Danish population-based cohort study. Cancer. doi: 10.1002/cncr.32580. 126:659-669, 2020. [DOI] [PubMed] [Google Scholar]
- 34. Children’s Oncology Group: Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers 2008. https://childrensoncologygroup.org/index.php/survivorshipguidelines.
- 35. National Comprehensive Cancer Network: NCCN guidelines: Adolescents and young adults with cancer, 2019. NCCN.org/patients.
- 36.Barthel EM, Spencer K, Banco D, et al. Is the adolescent and young adult cancer survivor at risk for late effects? It depends on where you look. J Adolesc Young Adult Oncol. 2016;5:159–173. doi: 10.1089/jayao.2015.0049. [DOI] [PubMed] [Google Scholar]
- 37.Parsons HM, Schmidt S, Harlan LC, et al. Young and uninsured: Insurance patterns of recently diagnosed adolescent and young adult cancer survivors in the AYA HOPE study. Cancer. 2014;120:2352–2360. doi: 10.1002/cncr.28685. [DOI] [PMC free article] [PubMed] [Google Scholar]