FIG 1.
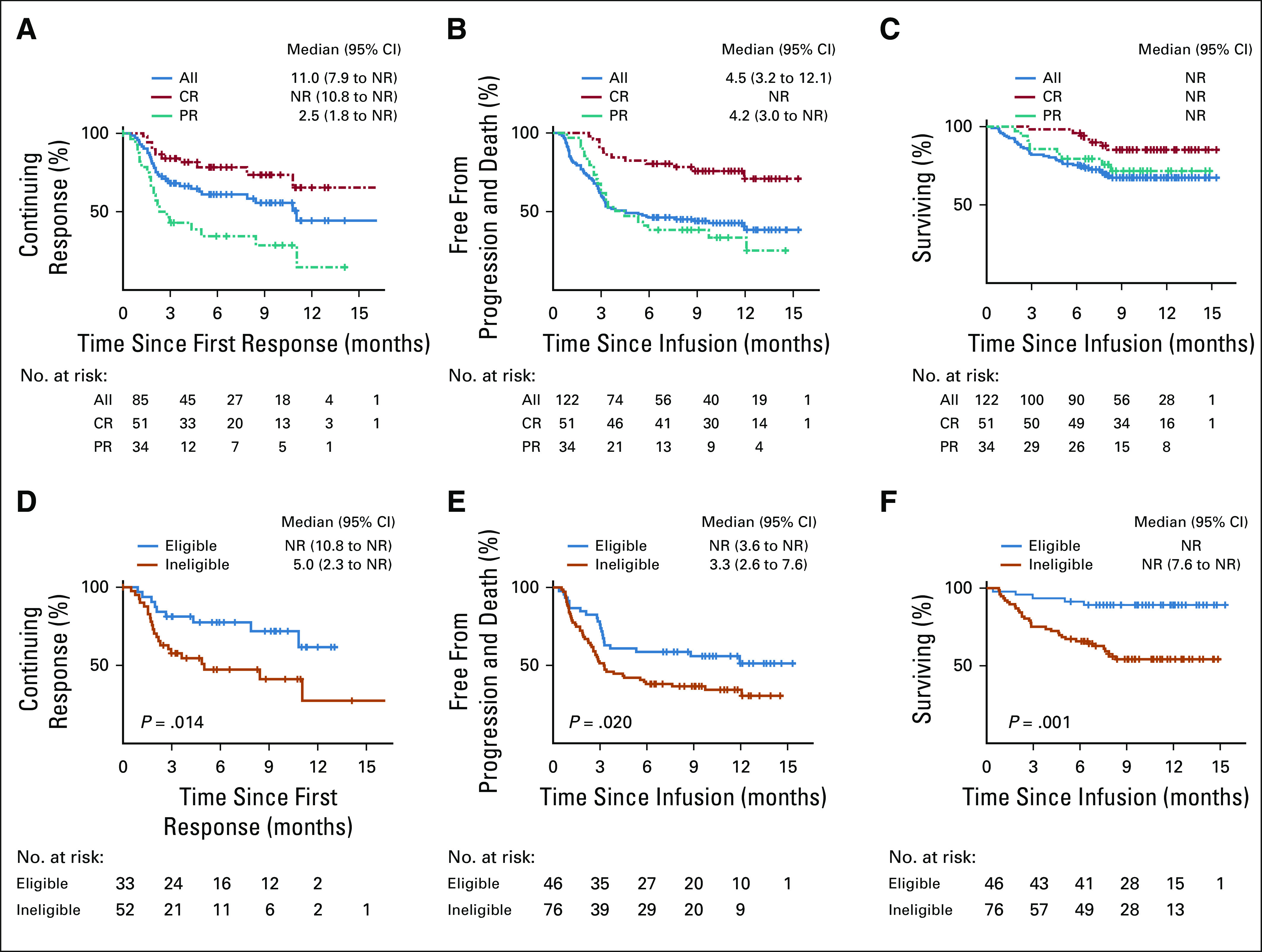
Efficacy outcomes of axicabtagene ciloleucel (axi-cel) overall and by ZUMA-1 eligibility. (A) Duration of response (DOR) curves for patients with overall response at first restage after chimeric antigen receptor (CAR) T-cell therapy. (B) Progression-free survival (PFS) curves for all patients. (C) Overall survival (OS) curves for all patients who underwent infusion of axi-cel. (D) DOR curves for patients who would have been eligible for ZUMA-1 (blue) and those who were ineligible for ZUMA-1 (orange). (E) PFS curves for patients who would have been eligible for ZUMA-1 (blue) and those who were ineligible for ZUMA-1 (orange). (F) OS curves for patients who would have been eligible for ZUMA-1 (blue) and those who were ineligible for ZUMA-1 (orange). All, all patients who underwent infusion of axi-cel; CR, complete response at first restage; NR, not reached; PR, partial response at first restage.
