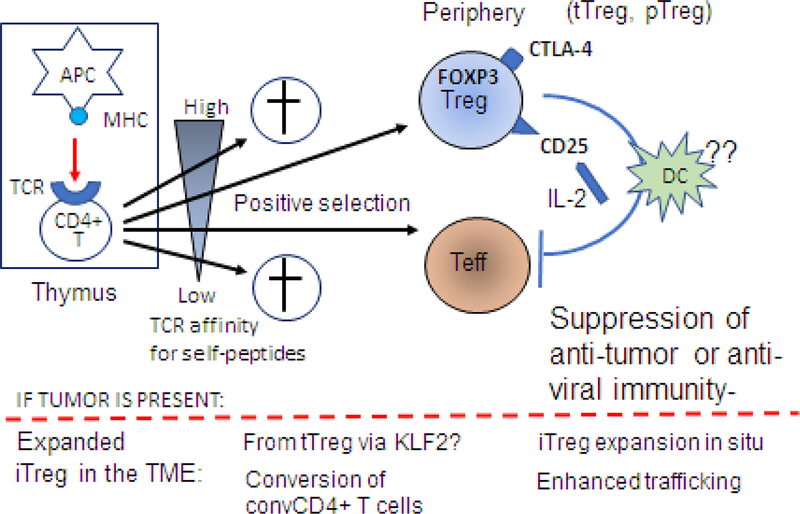
Figure 2.
Treg origin and development: Natural Treg are generated in the thymus from naïve CD4+CD8- thymocytes which interact with self-reactive thymic antigen-presenting cells (APCs) expressing self-peptide/MHC complexes. Stimulation of CD4+CD8- thymocytes by a T cell receptor (TCR) signal and continuous activation by IL-2 binding to CD25 (IL-2 receptor) leads to FOXP3 expression and differentiation into Treg. Note that thymocytes with the low affinity for self-peptides as well as those with very high affinity for self-peptides are eliminated. Only thymocytes with the high/intermediate affinity for self-peptides are positively selected for development and give rise to CD4+ effector T cells (Teff) or to CD4+FOXP3+ CD25+ tTreg and pTreg. In the periphery, Treg upregulate expression levels of CD25 and CTLA4. Upon interaction with an APC in the presence of IL-2, peripheral Treg become fully suppressive Treg that inhibit anti-tumor functions of Teff. It remains unclear how exactly this conversion happens, but it appears that iIn the presence of tumor-derived factors, pTreg and/or tTreg become inducible (i)Treg. The conversion of tTreg might require the KLF2 transcription factor. It is also possible that convTCD4+ T cells can undergo reprogramming to iTreg. This conversion is accompanied by an expansion of iTreg and their migration to the tumor site.