Fig. 1. Complementary network bioink 3D printing process.
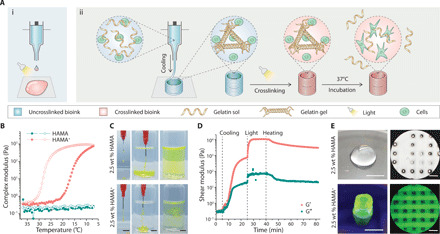
(A) (i) Schematic of the direct 3D printing of nonviscous, photocrosslinkable bioinks, which form droplets during extrusion and undergo spreading once deposited, even with light irradiation during the printing process. (ii) Schematic of the 3D printing of complementary network bioinks in three core steps: cooling-induced deposition mediated by the gelation of gelatin, photocrosslinking of the bioink, and thermal liberation of gelatin during incubation. (B) Oscillatory temperature sweep (between 37°C and 7°C at a rate of 5°C min−1; closed markers indicate cooling and open markers indicate heating) showing reversible cooling-induced gelation of HAMA+, while undoped HAMA exhibits a low viscosity across the monitored temperature range. (C) The injection of HAMA+ through a needle generates stable filaments in both air and PBS, while undoped HAMA does not (both formulations supplemented with dye for visualization). (D) Storage (G′) and loss (G″) moduli during an oscillatory time sweep of the 2.5 wt % HAMA+ bioink during stages of cooling (sharp temperature change from 37°C to 15°C), UV light exposure (10 mW cm−2), and heating back to 37°C. (E) Representative images of 3D printed tubular and lattice constructs using 2.5 wt % HAMA and HAMA+ (containing 5 wt % Fluorescein-gelatin). A frequency of 1.5 Hz and a strain of 1% were used for oscillatory tests. Scale bars, 5 mm (C and E, left) and 1 mm (E, right). Credit for all photographs in this figure: Liliang Ouyang, Imperial College London.
