Figure 1. Oleochemical Metabolism and Routes to 1-Octanol.
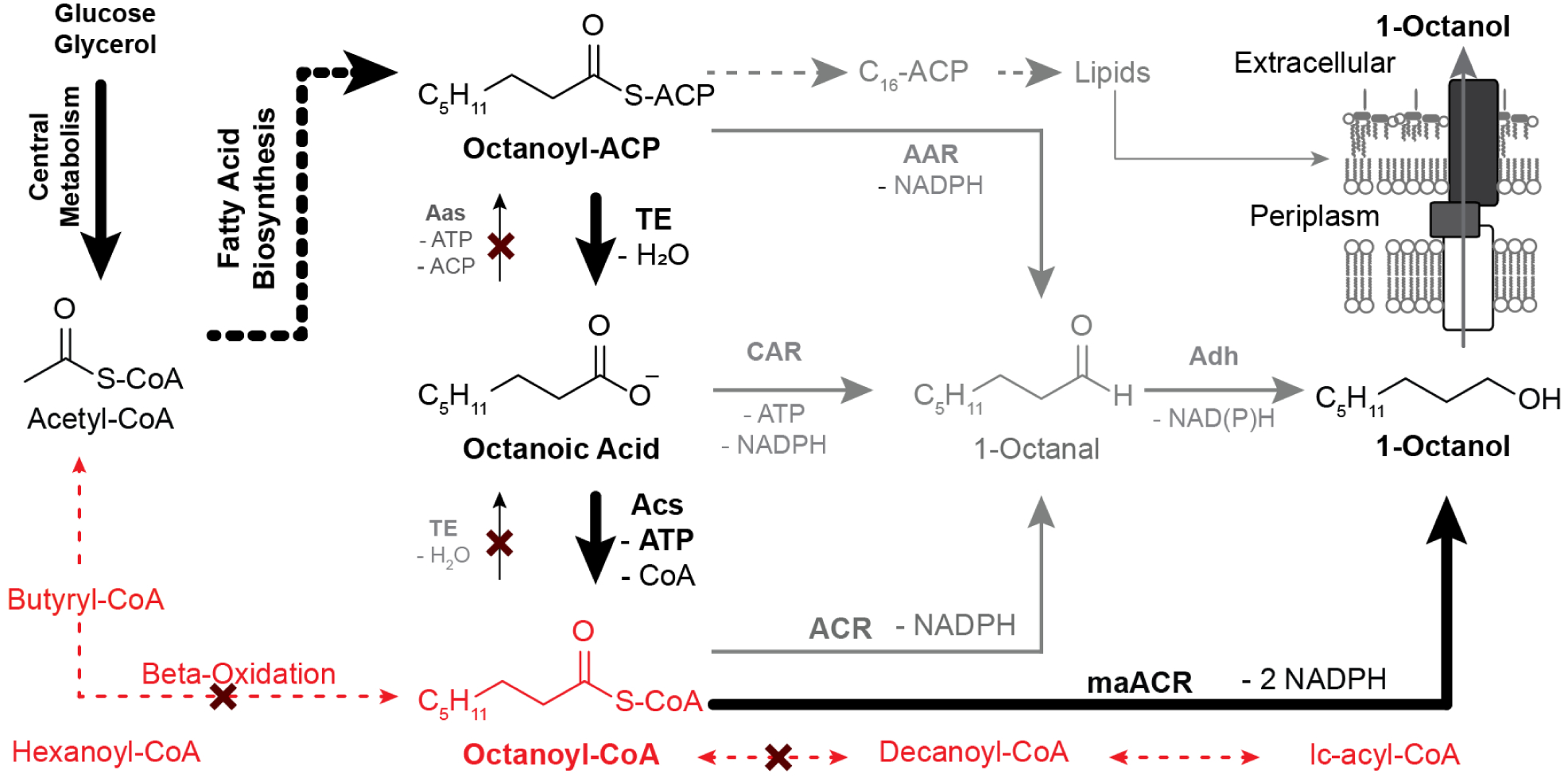
Oleochemicals are derived from acetyl-CoA that is generated in central metabolism from exogenous feedstocks (e.g. glucose, glycerol). Longer acyl-chains are synthesized via fatty acid biosynthesis (black hashed lines) or beta-oxidation (red hashed lines). The strategy applied in this project uses a heterologous thioesterase with activity towards octanoyl-ACP to divert flux from fatty acid biosynthesis to octanoic acid. Octanoic acid is then activated to octanoyl CoA by an acyl-CoA synthase (ACS). Beta oxidation is blocked such that a pool of octanoyl-CoA accumulates. Octanoyl-CoA is reduced twice by a dual-function acyl-CoA reductase (ACR) and aldehyde reductase (Adh) encoded by the Marinobacter aquaeolei MaACR. Alternative routes (in grey), including 1.) CAR mediated reduction of octanoic acid, 2.) direct reduction of octanoyl-ACP to octanal, and 3.) reduction of octanoyl-CoA to octanal by a distinct ACR were not pursued.
