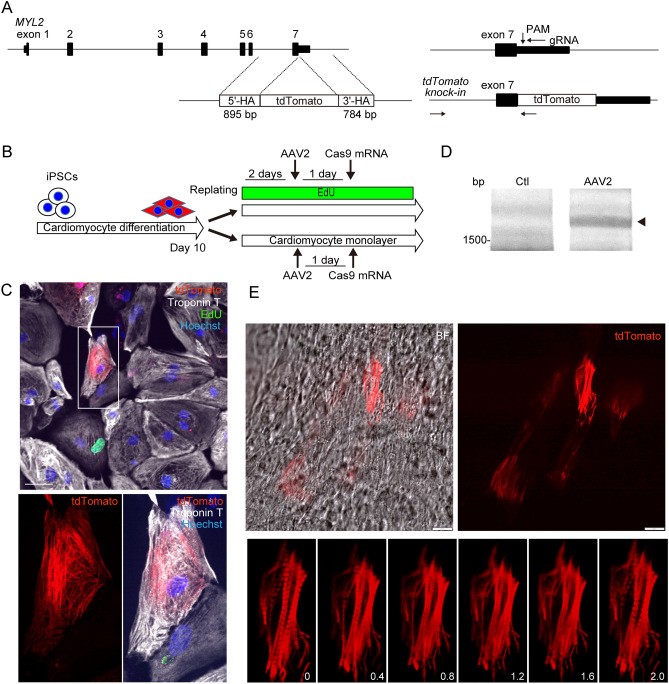
Figure 5.
AAV2 transduction followed by transient expression of Cas9 promotes targeted genome replacement in human monolayered cardiomyocytes differentiated from hiPSCs. (A) Genomic sequence of Human MYL2. gRNA was designed in an antisense direction targeting the PAM sequence located 19 bp downstream of the MYL2 stop codon. The repair template consists of the coding sequence of the tdTomato fluorescent reporter protein between the 895-bp 5′-terminal and the 784-bp 3′-terminal homology arms (5′-HA and 3′-HA), corresponding to the genomic sequence of the 3′-terminus of MYL2. Arrows indicate the locations of PCR primers to detect targeted integration after successful recombination. The forward primer to amplify the genomic sequence was designed outside of 5′-HA. (B) Experimental procedure of cardiomyocyte differentiation from hiPSCs and AAV2 transduction. hiPSCs were differentiated into cardiomyocytes using a monolayer protocol. Ten days after differentiation induction, hiPSC-CMs were replated into 96-well plates. EdU was added 2 days before AAV2 transduction. One day after transduction, cardiomyocytes were transfected with mRNA encoding Cas9, then continuously incubated with EdU. Five days after transduction, cells were fixed and immunostained. (C) A representative image of a tdTomato-positive, EdU (green)-negative, Troponin T (white)-positive hiPSC-CM is shown. Scale bar: 50 μm. (D) hiPSC-CMs were treated as in (B). Seven days after AAV2 transduction, genomic DNA was extracted and the targeted sequence was amplified by PCR using the primers indicated in (A). Original full image of the gel is presented in Supplementary Fig. 6E. (E) Contracting, monolayered, sheet-like hiPSC-CMs 14 days after differentiation were transduced with AAV2, then transfected with mRNA encoding Cas9. Scale bar: 20 μm. Lower panels show the serial consecutive fluorescent images obtained every 0.4 s.