Abstract
Changes in seawater chemistry due to anthropogenic uptake of CO2 by seawater results in a phenomenon termed ocean acidification. Ocean acidification has been predicted to substantially affect the exposure, behaviour, mobility and fate of toxicants with significant impacts on marine organisms. This study assessed the interactive effects of acidification and metal concentrations of Cd and Pb in the exoskeleton of the crab Dotilla fenestrata. Crabs were acutely exposed to varying concentrations of Cd (0.5, 0.75 and 1.00 mg/l), Pb (6.50, 8.50, and 10.50 mg/l) and Cd/Pb (4.50, 5.75 and 7.00 mg/l) and near-future pH of 7.2, 7.4 and 7.6 for 96 h and concentrations in the exoskeleton were analyzed using ICP-OES. Cadmium concentrations in the exoskeleton due to pH effects were in the order of 7.4 > 7.6 > 7.2, while concentrations in the exoskeleton exposed to pH 7.4 were significantly higher (ANOVA HSD: df 6; p < 0.01) compared to those of pH 7.2 and 7.6. Crabs exposed to varying Pb concentrations showed no common trend in Pb concentrations with varying pH. Concentrations of Cd and Pb in the exoskeleton of crabs exposed to combined Cd and Pb were significantly higher (ANOVA HSD: df 6; p < 0.01) at pH of 7.2 and 4.50 and 7.00 mg/l exposures. Crabs exposed to mixed metal concentrations showed elevated levels of Cd and Pb compared to those exposed to single metal due to their regulatory capacity when exposed to mixed metals.
Keywords: Environmental pollution, Environmental toxicology, Biological sciences, Biochemistry, Toxicology, Ocean acidification, ph, Cadmium, Lead, Dotilla fenestrata
Environmental pollution; Environmental toxicology; Biological sciences; Biochemistry; Toxicology; Ocean acidification; ph; Cadmium; Lead; Dotilla fenestrata.
1. Introduction
Globally, estuarine systems are progressively being transformed and endangered by the impacts of rising anthropogenic activities and global climate change (GCC) thereby exerting profound and diverse consequences on marine ecosystems (Vivier, 2010; Doney et al., 2011). Rising atmospheric CO2 remains a global concern due to its pervasive and irreversible consequences on ecological timescales (Council, 2011). Environmental factors like ocean acidification, rainfall patterns, the rising water temperature will considerably be affected by GCC according to IPCC forecast (Hoegh-Guldberg et al., 2014). Although the interactive effects of GCC with other anthropogenic stressors such as chemical contaminants are still not fully understood, there are concerns about the influential role GCC plays on the environmental concentration of contaminants (Gouin et al., 2013), as GCC is anticipated to have a significant effect on exposure, behaviour, fate, and release of toxicants (Noyes et al., 2009).
Ocean acidification is a key threat to marine biodiversity in estuarine and coastal ecosystems (Campbell et al., 2014; Ivanina and Sokolova, 2015) and is broadly considered to represent a significant threat to global marine biodiversity (Campbell et al., 2014). In the course of the past 650 000 years preceding the industrial revolution, concentrations of atmospheric CO2 ranged from 280 ppm (Gao et al., 2019). As a result, anthropogenic activities, mainly due to the burning of fossil fuel, industrialization and changes in land use (Hooper et al., 2013), the atmospheric concentration of CO2 has increased to about 409 ppm in 2018 and is currently increasing at a rate of ~0.5 % annually (Gao et al., 2019), approximately 100 times more rapid than any change in the past 650 000 years. The net effect of increasing the partial pressure of CO2 (pCO2) or hypercapnia of seawater leads to the shift in the pH and the carbonate ocean chemistry (Ivanina and Sokolova, 2015). The inorganic carbon system is an important chemical equilibrium in seawater chemistry and is generally responsible for controlling the seawater pH (Fabry et al., 2008). Dissolved inorganic carbon (DIC) exists in seawater in three main forms; bicarbonate ion (HCO3−), carbonate ion (CO32−), and aqueous carbon dioxide (CO2 (aq)) which also includes carbonic acid (H2CO3). About 88 % of the carbon forms HCO3− at the pH of 8.2, while 11 % is in the form of CO32−, and only about 1 % of the carbon forms dissolved CO2. Carbon dioxide dissolves in seawater to form H2CO3, and most of the H2CO3 quickly dissociates into a hydrogen ion (H+) and (HCO3−). The hydrogen ion then proceeds to react with CO32− to form bicarbonate.
Therefore, the resultant effect of seawater absorbing CO2 is increasing concentrations of H2CO3, HCO3−, and H+, and the concomitant decrease in the concentration of CO32−and lower pH (pH = -log[H+]). These reactions are fully reversible, and the basic thermodynamics of these reactions in seawater are well known (Millero et al., 2002; Fabry et al., 2008).
Elevated pCO2 of seawater (i.e. hypercapnia) could cause deleterious effects on marine organisms through reduced calcium carbonate (CaCO3) saturation, resulting in low calcification rates, and disturbance to acid-base (metabolic) physiology. Current studies also reveal the uptake of anthropogenic CO2 by the ocean, and the resultant alterations in the chemistry of seawater have adverse effects on many calcifying marine organisms, thereby causing modifications to trophic interactions, biodiversity and other ecosystem processes (Kleypas et al., 2005; Raven et al., 2005; Pascal et al., 2010).
Alterations in seawater chemistry as a result of ocean acidification can affect solubility, speciation and distribution of heavy metals in sediments and water, potentially affecting the toxicity of metals to marine organisms (Ivanina and Sokolova, 2015). Low pH increases the solubility of heavy metals and can cause metal desorption from the sediments and organic ligands, resulting in a higher influx of the dissolved metals into the water column (Ivanina and Sokolova, 2015). In line with this trend, elevated CO2 levels within the range of predicted near-future ocean acidification scenarios of ~700–1,500 μatm pCO2, increases heavy metal solubility and metal (Ni, Zn, and Fe) influx from the sediments into the water column (Breitbarth et al., 2010; Roberts et al., 2013). Also, mobilization of Cd, Cu, Pb and Zn into the water column increases at lowered pH of 7.5 and 6.5 depending on the binding strength between the metal and the sediment particles with the degree of mobilization dependent on the strength of the association (Riba et al., 2003).
The desorption of heavy metals from sediments into water column could be enhanced by the ocean acidification-induced reduction in biological calcification and dissolution of CaCO3, thus leading to the local increase in the concentrations of Ca2+ in the water, and also resulting in an enhanced release of heavy metal pollutants by competing with Ca2+ and H+ for their binding sites (Du Laing et al., 2009). Metal speciation strongly influences heavy metal bioavailability due to the free ionic forms usually being highly bioavailable (Paquin et al., 2000). Speciation of numerous metals that form strong complexes with OH and CO32− ions are predicted to be significantly affected by ocean acidification (Millero et al., 2009). Concentrations of OH− and CO32− reduce with ocean acidification resulting in higher concentrations of the most bioavailable free ionic form of these metals (Ivanina and Sokolova, 2015). Copper and nickel are examples of metals that form strong complexes with CO32−; therefore, ocean acidification-induced reduction in CO32− levels will invariably cause an increase in free Cu2+ and Ni2+ concentrations (from the present-day ~ 8 % and 4 % to ~32 % and 13 % by the year 2250 for Cu2+ and Ni2+, respectively) (Millero et al., 2009). Free iron concentrations (Fe2+) are expected to increase from the current 66 %–90 % by the year 2250, while the speciation of other heavy metals is projected to be less affected by ocean acidification (Ivanina and Sokolova, 2015). The proportion of free Pb2+ is expected to increase from ~3 % to ~6 % by the year 2250 (pH 7.4) (Millero et al., 2009), while speciation of the metals which predominantly form complexes with chloride (e.g. Cd2+and Hg2+) are insensitive to ocean acidification (Millero et al., 2009).
From recent studies on the effects of seawater pCO2/pH on uptake and accumulation of heavy metals, an intricate pattern which cannot be easily predicted from the chemical models of metal speciation and ligand binding is depicted (Ivanina and Sokolova, 2015). The effects of pCO2/pH on uptake, accumulation and toxicity of metals are dependent on the species, organism's life stage and the OA levels rather than the predicted concentrations of the free metals in seawater (Ivanina and Sokolova, 2015). Uptake and accumulation of Cd, one of the most studied metals about ocean acidification-metal interactions could serve as a useful reference illustration for species and environment-dependent variability of responses to pCO2. Cadmium speciation, unlike Cu and Fe, is independent of pH and pCO2 (Ivanina and Sokolova, 2015).
The physiological effects of OA in many marine organisms have been extensively studied (Das and Mangwani, 2015), but the potential for OA to interact with other environmental stressors remains poorly understood (Crain et al., 2008). Till date, such studies have focused on combining OA with either temperature, salinity or hypoxia (Lewis et al., 2016). Of particular interest for environmental assessment, however, is the understanding of how near-future OA will change the behaviour and bioavailability of persistent marine contaminants, notably heavy metals. Studies by Lewis et al. (2016), revealed that near-future OA scenarios significantly increases the sub-lethal toxicity responses of two key coastal marine invertebrates, namely mussels (Mytilus edulis) and urchins (Paracentrotus lividus) to relevant concentrations of copper in the marine environment. Copper-induced damage to DNA of both marine invertebrates was significantly greater when the animals were exposed to nominal 0.1 μm copper under OA (high pCO2/low pH) conditions compared with animals exposed under extant pCO2 levels (Lewis et al., 2016).
Crabs are capable of taking up and accumulating trace metals in their tissues and are, therefore a suitable bioindicator for environmental contamination assessment (Kumar et al., 2000; Bastami et al., 2012). Dotilla fenestrata (Hilgendorf, 1869), the sand-bubbler crab, is a small species and is about 1cm across the carapace (Dray and Paula, 1998; Gherardi et al., 2002; Flores et al., 2005). They belong to the Ocypodidae family of brachyuran crabs and are widely distributed along the East African coast from Kenya to South Africa and also found in Madagascar and The Comoro Islands (Hartnoll, 1973; Bulcao and Hodgson, 2012). They are burrowing decapod crustaceans and occur abundantly on soft sediment shores in tropical and sub-tropical climates (Maitland, 1986; Bulcao and Hodgson, 2012). Sand bubbler crabs are distributed mainly in the north of Durban, South Africa (29° 52′ S; 31° 04′E), although small numbers are found in warm temperate regions as far south of the Breede River estuary (Day, 1974, 1981; Rius et al., 2010). Dotilla fenestrata plays important ecological roles like other burrowing crustaceans as a deposit feeder and bioturbator within its habitat (Flores et al., 2005). Its bioturbation function, i.e. the process that is responsible for a rapid rate of sediment turnover that results in a change in the physical, chemical and biological characteristics of the sediment (Branch and Pringle, 1987; Dray and Paula, 1998; Flores et al., 2005) has been shown to affect the productivity of sandy shores and changing of meiofaunal communities (Flores et al., 2005). This study aims to determine the effects of near-future coastal acidification on the concentrations of heavy metal (Cd and Pb) in the exoskeleton of crab as a result of increasing coastal acidity.
2. Materials and methods
2.1. Crab sampling
Dotilla fenestrata (N = 540; 7 ± 1 mm carapace width) collected from Durban Bay Harbour, KwaZulu-Natal in line with the recommended ethical and governmental requirements, were cleaned with filtered seawater to remove debris and washed with 30 % artificial seawater to eliminate unwanted contaminants. They were subsequently acclimated in a constant temperature room at 18 °C and 32 psu, controlled photoperiodic duration of 12L:12D with a pH of 8.1 for 72 h.
2.2. Experimental setup
The acute exposures of crabs were carried out according to the standard methodology such as those of the FAO (Ward and Parrish, 1982; Reisch and Oshida, 1987) and the American Public Health Association (Apha, 1992). Prior to exposures and preparations of stock solution, all glassware was soaked in 10 % nitric acid and rinsed thoroughly with double distilled water and deionized water. A three by three experimental grid design was adopted, and a range-finder test was run to select applicable metal concentrations for the crabs to be exposed to in 96 h. Crabs were exposed to three varying metal concentrations and pH in triplicates per combined concentrations of metal and pH by bubbling CO2 using an automated CO2 system into glass tanks containing 5 L of (Fatoki and Mathabatha, 2001) homogenized stock solutions of sub-lethal concentrations of Cd (0.5, 0.75 and 1.0 mg/l), Pb (6.5, 8.5 and 10.5 mg/l) and Cd & Pb (4.5 (0.50 Cd & 4.00 Pb), 5.75 (0.75 Cd & 5.00 Pb)and 7.0 (1.00 Cd & 6.00 Pb) mg/l) freshly prepared by dissolving the appropriate analytical grade of metal salts CdCl2.5H2O for Cd, and Pb(NO3)2 for Pb in deionized water with standard glass flasks. These sub-lethal concentrations fall between the range of Cd and Pb concentrations obtained by Fatoki and Mathabatha (2001) in water and sediment from East London, and Port Elizabeth Harbours in South Africa and represent about 1000 % order of magnitude above the South African marine water quality guideline target values (Cd 4.0 μg/g and Pb 12.0 μg/g). Stock solutions were acidified by the controlled bubbling of CO2 into stock solution (Chapman, 1978) to obtain varying pH groups of 7.2, 7.4 and 7.6 to simulate predicted near-future coastal pH. Tanks were covered with plastic lids to minimize evaporation and small holes bored through the centre to allow for bubbling of air and CO2 throughout the experiment. Test solutions were set up and running 24 h before the introduction of crabs. The experiment was conducted in a constant temperature room at 18 °C and 32 psu and a controlled photoperiodic duration of 12L: 12D. Before each experiment, 20 active crabs were gently introduced into each tank. Water quality parameters were monitored periodically throughout the 96-hour duration of the experiment to ensure that all variables were within experimental limits. At the end of the experiment, haemolymph was extracted for osmolality determination, and the remaining crabs were stored in a freezer for metal analysis.
2.3. Sample preparation and analysis
Crabs were thawed and dissected for the tissue – exoskeleton (as the tissue compartment with the most significant metal accumulation (Adeleke, 2017)). Dissected tissue was weighed, and oven-dried at 50 °C to constant weight for at least 48 h. About 0.5g of homogenized dried tissues were crushed to uniform particle size with a lab porcelain mortar and pestle and digested in 20 ml concentrated AR grade nitric acid for at least 24 h. Subsequently, the digested samples were mixed with 10 ml of concentrated AR grade nitric and perchloric acid (4:1), heated on a hot plate at 120 °C and subsequently made up to 20 ml by adding 20 ml solution of Milli-Q water with 20 % nitric acid and filtered with Whatman filter paper (Sudharsan et al., 2012). Heavy metal concentrations were determined using ICP-OES, Perkin Elmer optima 5300 DV and crab tissues were analyzed for Cd and Pb using crab paste (LGC 7164) as certified reference material to test for analytical accuracy of the metals as represented in Table 1.
Table 1.
Measured and certified values of trace metal concentrations in µg/g certified reference materials.
| Metal |
Cadmium (Cd) µg/g |
Copper (Cu) µg/g |
Lead (Pb) µg/g |
Zinc (Zn) µg/g |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Measured | Certified | % recovery | Measured | Certified | % recovery | Measured | Certified | % recovery | Measured | Certified | % recovery |
| Crab | 8.98 ± 0.08 | 9.2 ± 0.48 | 97.61 | 23.8 ± 0.33 | 20.1 ± 2.4 | 118.41 | 0.49 ± 1.39 | 0.47 ± 0.005 | 104.26 | 66 ± 0.43 | 56.8 ± 5.5 | 110.00 |
| Sediment | 7.29 ± 1.87 | 5.52 ± 0.75 | 132.07 | 109 ± 2.57 | 116 ± 22.0 | 93.97 | 172 ± 3.82 | 187 ± 22.0 | 91.98 | 907 ± 55.7 | 861 ± 165 | 105.22 |
| Water | 105 ± 23.7 | 101 ± 2.0 | 103.96 | 180 ± 45.6 | 190 ± 4.0 | 94.74 | 203 ± 32.9 | 196 ± 3.0 | 103.57 | 50.2 ± 17.7 | 55.0 ± 0 | 94.55 |
2.4. Statistical analysis
Main effects analysis of variance (ANOVA) and pairwise comparisons analysis using Tukey's HSD test were used to test for a statistical difference in mean metal concentrations in crab tissue due to combined effects of pH and metal. Pearson's correlation coefficient was used to determine the relationship between mean metal concentrations in the crab tissue and varying pH using Statistica 13.0 software program.
3. Results
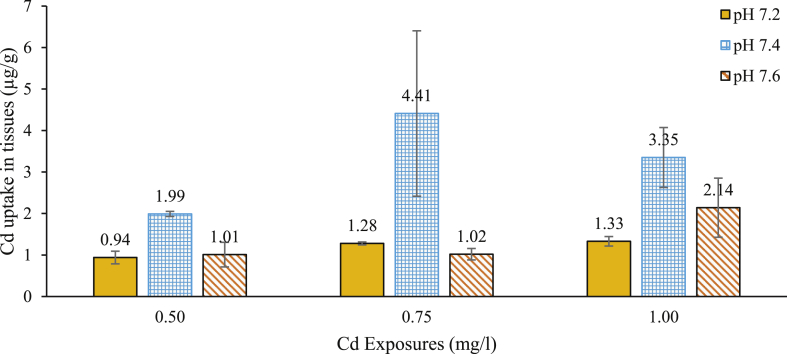
Mean cadmium concentrations in the exoskeleton of crabs exposed to a different combination of acute exposures of Cd and pH media occurred in the order of 7.4 > 7.6 > 7.2 (Figure 1 and Table 2). Cadmium concentration in the exoskeleton of crabs exposed to pH 7.4 with Cd 0.50 mg/l concentration was significantly higher (ANOVA HSD: df 6; p < 0.01) compared with those exposed to pH 7.2 and 7.6. There was, however, no significant difference (ANOVA HSD: df 6; p > 0.05) between the Cd levels in the exoskeleton of crabs in media pH 7.2 and 7.6 (Table 2).
Figure 1.
Mean Cd concentrations (μg/g ± SD) in the exoskeleton of D. fenestrata after 96 h acute exposure to 0.50, 0.75 and 1.00 mg/l Cd solutions.
Table 2.
Main Effect ANOVA (Tukey HSD Test) (pH effect) of Crab exoskeleton exposed to different pH and metal (Cd, Pb and Cd/Pb) combinations. n = 81 (Mean value (μg/g) ± SD). (∗) denotes significant difference (p < 0.05), (∗∗) denotes highly significant difference (p < 0.01).
| Metal Exposures | pH | Cd df = 6 |
Pb df = 6 |
Cd (Cd/Pb) df = 6 |
Pb(Cd/Pb) df = 6 |
|---|---|---|---|---|---|
| (Cd 0.50 mg/l) | 7.2 | 0.94 ± 0.15 | 272.54 ± 68.88 | 5.14∗∗ ± 1.15 | 282.59∗∗ ± 3.79 |
| (Pb 6.50 mg/l) | 7.4 | 1.99∗∗ ± 0.06 | 333.15 ± 62.75 | 2.02 ± 0.17 | 171.13 ± 27.74 |
| (Cd/Pb 4.50 mg/l) | 7.6 | 1.01 ± 0.30 | 365.50 ± 3.46 | 1.60 ± 0.03 | 166.21 ± 3.58 |
| (Cd 0.75 mg/l) | 7.2 | 1.28 ± 0.03 | 390.90 ± 3.04 | 2.80 ± 0.42 | 153.80 ± 23.39 |
| (Pb 8.50 mg/l) | 7.4 | 4.41∗ ± .99 | 370.70 ± 48.35 | 2.10 ± 0.08 | 412.70∗∗ ± 20.88 |
| (Cd/Pb 5.75 mg/l) | 7.6 | 1.02 ± 0.14 | 315.24 ± 68.40 | 2.50 ± 0.13 | 153.30 ± 3.22 |
| (Cd 1.00 mg/l) | 7.2 | 1.33 ± 0.11 | 290.70∗∗ ± 1.49 | 3.60∗∗ ± 0.34 | 379.5∗∗ ± 76.40 |
| (Pb 10.50 mg/l) | 7.4 | 3.35∗ ± 0.72 | 213.86 ± 17.43 | 3.00 ± 0.10 | 163.80 ± 7.40 |
| (Cd/Pb 7.00 mg/l) | 7.6 | 2.14 ± 0.71 | 340.40∗∗ ± 3.37 | 2.60 ± 0.17 | 189.60 ± 12.36 |
| Background Concentrations | 8.1 | 0.42 ± 0.00 | 2.43 ± 0.10 |
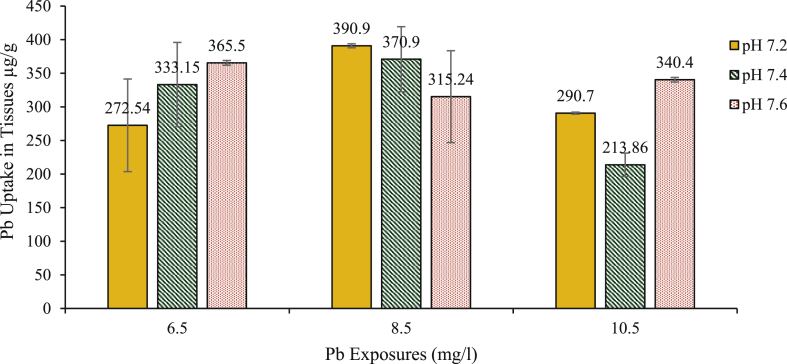
Bioconcentration of Pb in the crabs differed with increasing concentrations of Pb in the water medium and showed different trends with varying pH (Figure 2). Concentration of Pb in the exoskeleton of crabs exposed to concentrations of 10.50 mg/l Pb with pH 7.6 was significantly higher (ANOVA HSD: df 6; p < 0.01) compared to those at pH 7.4/10.50 mg/l, while those exposed to pH 7.2/10.50 mg/l were significantly higher (ANOVA HSD; df 6; p < 0.01) compared to those exposed to pH 7.4/10.50 mg/l. Crabs exposed to Pb concentrations of 6.50 mg/l and 8.50 mg/l showed no significant variation (ANOVA HSD; df 6; p > 0.05) in the exoskeleton's levels of Pb with varying pH (see Figure 2 and Table 2).
Figure 2.
Mean Pb concentrations (μg/g ± SD) in the exoskeleton of D. fenestrata after 96 h acute exposure to 6.50, 8.50 and 10.50 mg/l Pb solutions.
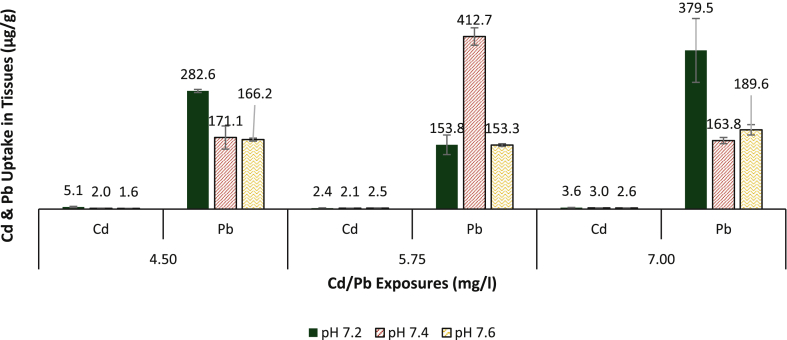
Bioconcentration of Cd and Pb in the exoskeleton of crabs exposed to mixed metal Cd/Pb differed with varying pH compared to those exposed to either Cd or Pb only (Figures 1, 2, and 3). Cadmium and Pb concentrations in the exoskeleton of crabs exposed to media concentrations of 4.50 mg/l and 7.0 mg/l of mixed Cd/Pb with pH 7.2 were significantly higher (ANOVA HSD: df 6; p < 0.01) compared with those at pH 7.4 and 7.6, while Pb concentration in the exoskeleton of crabs exposed to 5.75 mg/l Cd/Pb with pH 7.4 was significantly higher (ANOVA HSD: df 6; p < 0.01) than those at pH 7.2/5.75 mg/l and pH 7.6/5.75 mg/l Cd/Pb concentrations. There was no significant difference (ANOVA HSD: df 6; p > 0.05) in Cd concentration in the exoskeleton of crabs exposed to Cd/Pb concentration of 5.75 mg/l with different pH (Figure 3 and Table 2).
Figure 3.
Mean Cd/Pb concentrations (μg/g ± SD) in the exoskeleton of D. fenestrata after 96 h acute exposure to 4.50, 5.75 and 7.00 mg/l Cd/Pb solutions.
The Pearson's Correlation coefficient between the exoskeleton of the crabs exposed to combinations of varying pH of 7.2, 7.4 and 7.6 and concentrations of heavy metals (Cd, Pb and Cd/Pb) did not show any significant correlation (p > 0.05) in this study (Table 3).
Table 3.
Pearson's Correlation Coefficient (R) between of the crab exoskeleton exposed to different pH and metal (Cd, Pb and Cd/Pb) combinations. n = 9 (∗) denotes significant correlation (p < 0.05), (∗∗) denotes high significant correlation (p < 0.01).
| Exposures | pH 7.2 | pH 7.4 | pH 7.6 |
|---|---|---|---|
| (Cd 0.50 mg/l) | 0.96 | 0.81 | 0.91 |
| (Cd 0.75 mg/l) | 0.96 | 0.83 | 0.89 |
| (Cd 1.00 mg/l) | 0.73 | 0.67 | 0.81 |
| (Pb 6.50 mg/l) | 0.11 | 0.23 | 0.15 |
| (Pb 8.50 mg/l) | 0.17 | 0.15 | 0.21 |
| (Pb 10.50 mg/l) | 0.74 | 0.85 | 0.67 |
| (Cd/Pb 4.50 mg/l) | 0.26 | 0.31 | 0.41 |
| (Cd/Pb 5.75 mg/l) | 0.72 | 0.65 | 0.92 |
| (Cd/Pb 7.00 mg/l) | 0.40 | 0.60 | 0.33 |
4. Discussion
Ocean/coastal acidification is a developing concern in marine ecosystems due to rising atmospheric CO2 concentrations and projected to affect speciation of heavy metals considerably thus resulting to the high bioavailability of the free ionic metals (Ivanina and Sokolova, 2015). Crustaceans accumulate trace metals in their tissues with high variability across different metals (Rainbow, 2007). Current studies on the effects of OA on uptake and concentrations of heavy metals reveal a complex form that is difficult to predict from metal speciation and ligand binding chemical models hence, the effects of PCO2/OA on bioaccumulation of metals and toxicity are mainly reliant on species, organism life stage and level of acidification rather than the levels of predicted bioavailability of metals in seawater (Ivanina and Sokolova, 2015). This study shows that at pH 7.4, mobilization and concentrations of Cd in the exoskeleton of D. fenestrata increases significantly compared to pH of 7.2 and 7.6. Concentrations of Cd in the exoskeleton of the crabs were not directly proportional to elevated CO2 concentration in the water column (Figure 1). These results corroborate those of (Millero et al., 2009) Millero et al. (2009) which showed metals such as Cu+, Cd2+, and Hg2+ that form strong complexes with chloride experience little or no alteration in speciation as a result of chloride concentration not being affected by lowering pH. Cadmium bioconcentrations in marine organisms represent a good reference for species and environmentally dependent hypercapnia variability, as Cd is a widely studied metal with regards to metal-OA interactions (Ivanina and Sokolova, 2015). Cadmium speciation is not dependent on pH and PCO2 compared to Cu and Fe, hence the bioavailability of free Cd ions are not the major factor influencing Cd uptake by organisms, instead of the interactions of complex factors which are largely dependent on species and levels of OA (Ivanina and Sokolova, 2015) (see Table 4). Uptake of Cd from the water column into mantle tissues of marine bivalves Mercenaria mercenaria and Crassostrea virginica increased with increasing levels of CO2 climaxing at intermediate PCO2 (~800 μatm) in oysters and extreme PCO2 (~2,000 μatm) in clams (Götze et al., 2014). The observed changes probably showed the effects of PCO2 on physiological uptake and distribution of Cd due to the suppression of Cd accumulation in clams as a result of high PCO2 isolated in their mantle (Ivanina et al., 2013). Uptake of Cd from sediment in clam Ruditapes philippinarum and an amphipod Corophium volutator juveniles were independent of PCO2 (López et al., 2010; Roberts et al., 2013). Also, elevated PCO2 reduced Cd uptake by tissue compartments of sea anemone Anemonia viridis (Horwitz et al., 2014). Therefore, a comprehensive biochemical model that can describe the effects of OA on metal bioavailability and toxicity to aquatic organisms is still doubtful due to metal diversity and hypercapnia interactions combined with the lack of a strong correlation between the PCO2-dependent metal speciation and uptake (Ivanina and Sokolova, 2015).
Table 4.
Percentage of metal forms of Cd and Pb in seawater due to the effects of pH and time at 25 °C and salinity of 35psu (Caldeira and Wickett, 2003).
| Year |
2000 |
2050 |
2070 |
2085 |
2100 |
2150 |
2200 |
2250 |
|
|---|---|---|---|---|---|---|---|---|---|
| pH |
8.1 |
8.0 |
7.9 |
7.8 |
7.7 |
7.6 |
7.5 |
7.4 |
|
| MetalSpecies | |||||||||
| Chloride Dominated | Cd2+ | 20.15 | 201.17 | 20.18 | 20.19 | 20.20 | 20.21 | 20.21 | 20.22 |
| CdCl+ | 43.71 | 43.75 | 43.78 | 44.10 | 43.82 | 43.80 | 43.85 | 43.86 | |
| CdCl2 | 27.70 | 27.72 | 27.74 | 28.07 | 27.77 | 27.78 | 27.79 | 27.79 | |
| CdCl3– | 7.95 | 7.95 | 7.96 | 7.97 | 7.96 | 7.97 | 7.97 | 7.97 | |
| Transition/Mixed | Pb2+ | 2.89 | 3.29 | 3.70 | 4.13 | 4.56 | 4.99 | 5.39 | 5.77 |
| PbOH+ | 4.24 | 3.83 | 3.40 | 3.03 | 2.66 | 2.31 | 1.98 | 1.68 | |
| PbCO3 | 13.09 | 14.86 | 16.74 | 18.68 | 20.63 | 22.54 | 24.37 | 26.07 | |
| PbCl+ | 59.03 | 54.53 | 49.72 | 44.71 | 39.64 | 34.65 | 29.88 | 25.43 | |
| PbCl2 | 14.09 | 16.00 | 18.02 | 20.10 | 22.21 | 24.60 | 26.23 | 28.06 | |
| PbCl3- | 6.40 | 7.27 | 8.19 | 9.14 | 10.09 | 11.03 | 11.93 | 12.76 | |
Lead concentration in the crab's exoskeleton in this study did not follow a defined order as accumulation in the exoskeleton varies with levels of Pb exposures and pH. While accumulation in crabs exposed to 6.50 mg/l Pb concentrations demonstrated increased Pb accumulation with increasing pH, those exposed to 8.50 mg/l accumulated more Pb with decreasing pH and those exposed to 10.50 mg/l exhibited an intermediate uptake and distribution behaviour to those exposed to 6.50 and 8.50 mg/l Pb concentrations. This could be due to the effects of the transitional behaviour of Pb, which enables it to form significant complexes with both Cl− and CO32− (see Table 4). Therefore, as pH decreases, the free ion form of Pb increases by ~ 10% resulting in a great increase in its complexation with Cl− (15% among PbCl, PbCl2, and PbCl3) (Millero et al., 2009). The physiology of the crab as it relates to absorption capacity, metabolism and modification of regulatory capacity when exposed to Pb only (Núñez-Nogueira et al., 2012) might also play a role. Many studies on the accumulation of Pb in marine organisms have broadly shown that uptake and bioaccumulation of Pb are mainly dependent on the level of bioavailability (Chinni et al., 2000). However, elevated concentration of Pb was observed in the exoskeleton of the crabs exposed to a metal mixture of Cd and Pb (Figure 3). This observation corroborates the findings of Núñez-Nogueira et al. (2012), who observed a significant elevation in body concentrations of Pb in shrimp Penaeus vannamei exposed to the mixed treatment of Pb and other metals. Elevated body concentration was detected when different metals competed at the same time, seemingly due to synergic effects stimulated by the occurrence of other metals in the water column. It can be inferred from the results that exposure of crabs to metal mixture aids metal uptake from the water column in several orders of magnitude, and body regulatory capacity seems to be compromised with combined metal uptake. Several studies have also demonstrated metal regulatory capacities of many marine crustaceans when exposed to mixed metal concentrations; Panulirus inflatus (Paez-Osuna et al., 1995), Macrobrachium malcolmsonii and Penaeus indicus (Vijayram and Geraldine, 1996) and Litopenaeus vannamei exhibited regulation to specific metal exposure levels of 0.2 mg/l (Wu and Chen, 2005). As observed from this study, crabs exposed to mixed metal (Cd/Pb) concentrations mostly have significantly elevated concentrations of Cd and Pb at pH of 7.2 compared to those at pH 7.4 and 7.6 except for crabs exposed to 5.75 mg/l water concentrations of Cd/Pb.
5. Conclusion
Ocean acidification affects the dynamics of heavy metals in seawater and will lower the pH of estuarine waters, thus altering the biogeochemical processes in these systems resulting in greater changes in metal speciation. The solubility of various metals in seawater is mainly dependent on pH and is affected by changes in speciation, which result in modifications to behaviour and release the fate of metals in the marine environment. The combined effects of these factors are mostly responsible for bioavailability, uptake, accumulation and toxicity of metals to aquatic organisms. This study shows that Cd uptake and accumulation in crab tissues were significantly elevated at pH of 7.4 and not directly proportional with decreasing pH, indicating that Cd mobilization in the water column and subsequent uptake by crabs was significantly higher at pH 7.4 compared to pH of 7.2 and 7.6. Lead uptake is dependent on bioavailability levels and the presence of other competing metals while accumulation in crab tissues is also dependent on regulatory physiology of the organism.
Declarations
Author contribution statement
Babatunde Ayoade Adeleke: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Deborah Robertson-Andersson, Gan Moodley: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by NRF South Africa and Gay Langmuir.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adeleke B.A. The Combined Effects of Near-Future Ocean Acidification and Varying Concentrations of Heavy Metals in Dotilla Fenestrata (Sand Bubbler Crab) in Durban Harbour, Richards Bay Harbour and Mlalazi Estuary, South Africa. 2017. Master’s Thesis. [Google Scholar]
- Apha A. WPCF (American public Health association, American waterworks association, water pollution control federation)(1992) standard methods for the examination of water and wastewater. Stand. Methods Exam. Water Wastewater. 1992;17 [Google Scholar]
- Bastami A., Khoei J., Esmailian M. Bioaccumulation of heavy metals in sediment and crab, Portunus pelagicus from Persian Gulf, Iran. Middle East J. Sci. Res. 2012;12(6):886–892. [Google Scholar]
- Branch G., Pringle A. The impact of the sand prawn Callianassa kraussi Stebbing on sediment turnover and on bacteria, meiofauna, and benthic microflora. J. Exp. Mar. Biol. Ecol. 1987;107(3):219–235. [Google Scholar]
- Breitbarth E., Bellerby R., Neill C., Ardelan M., Meyerhöfer M., Zöllner E., Croot P., Riebesell U. Ocean acidification affects iron speciation during a coastal seawater mesocosm experiment. Biogeosciences (BG) 2010;7(3):1065–1073. [Google Scholar]
- Bulcao C., Hodgson A. Activity and feeding of Dotilla fenestrata (Brachyura: Ocypodidae) in a warm, temperate South African estuary. Afr. J. Aquat. Sci. 2012;37(3):333–338. [Google Scholar]
- Caldeira K., Wickett M.E. Oceanography: anthropogenic carbon and ocean pH. Nature. 2003;425(6956):365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- Campbell A.L., Mangan S., Ellis R.P., Lewis C. Ocean acidification increases copper toxicity to the early life history stages of the polychaete Arenicola marina in artificial seawater. Environ. Sci. Technol. 2014;48(16):9745–9753. doi: 10.1021/es502739m. [DOI] [PubMed] [Google Scholar]
- Chapman G.A. Toxicities of cadmium, copper, and zinc to four juvenile stages of chinook salmon and steelhead. Trans. Am. Fish. Soc. 1978;107(6):841–847. [Google Scholar]
- Chinni S., Khan R., Yallapragada P. Oxygen consumption, ammonia-N excretion, and metal accumulation in Penaeus indicus postlarvae exposed to lead. Bull. Environ. Contam. Toxicol. 2000;64(1):144–151. doi: 10.1007/s001289910022. [DOI] [PubMed] [Google Scholar]
- Council N.R. National Academies Press; 2011. Climate Stabilization Targets: Emissions, Concentrations, and Impacts over Decades to Millennia. [Google Scholar]
- Crain C.M., Kroeker K., Halpern B.S. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 2008;11(12):1304–1315. doi: 10.1111/j.1461-0248.2008.01253.x. [DOI] [PubMed] [Google Scholar]
- Das S., Mangwani N. Ocean acidification and marine microorganisms: responses and consequences. Oceanologia. 2015;57(4):349–361. [Google Scholar]
- Day J. The ecology of Morrumbene estuary, Moçambique. Trans. Roy. Soc. S. Afr. 1974;41(1):43–97. [Google Scholar]
- Day J. Estuarine Ecology with Particular Reference to Southern Africa. 1981. Summaries of current knowledge of 43 estuaries in southern Africa; pp. 251–329. [Google Scholar]
- Doney S.C., Ruckelshaus M., Duffy J.E., Barry J.P., Chan F., English C.A., Galindo H.M., Grebmeier J.M., Hollowed A.B., Knowlton N. 2011. Climate Change Impacts on marine Ecosystems. [DOI] [PubMed] [Google Scholar]
- Dray T., Paula J. Ecological aspects of the populations of the crab Dotilla fenestrata (Hilgendorf, 1869)(Brachyura: Ocypodidae), in the tidal flats of Inhaca Island (Mozambique) J. Nat. Hist. 1998;32(10-11):1525–1534. [Google Scholar]
- Du Laing G., Rinklebe J., Vandecasteele B., Meers E., Tack F.M. Trace metal behaviour in estuarine and riverine floodplain soils and sediments: a review. Sci. Total Environ. 2009;407(13):3972–3985. doi: 10.1016/j.scitotenv.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Fabry V.J., Seibel B.A., Feely R.A., Orr J.C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES (Int. Counc. Explor. Sea) J. Mar. Sci. 2008;65(3):414–432. [Google Scholar]
- Fatoki O., Mathabatha S. An assessment of heavy metal pollution in the East London and Port Elizabeth harbours. Water SA. 2001;27(2):233–240. [Google Scholar]
- Flores A.A., Abrantes K.G., Paula J. Estimating abundance and spatial distribution patterns of the bubble crab Dotilla fenestrata (Crustacea: Brachyura) Austral Ecol. 2005;30(1):14–23. [Google Scholar]
- Gao K., Beardall J., Häder D.P., Hall-Spencer J.M., Gao G., Hutchins D.A. Effects of ocean acidification on marine photosynthetic organisms under the concurrent influences of warming, UV radiation and deoxygenation. Front. Mar. Sci. 2019;6:322. [Google Scholar]
- Gherardi F., Russo S., Lazzara L. The function of wandering in the sand-bubbler crab, Dotilla fenestrata. J. Crustac Biol. 2002;22(3):521–531. [Google Scholar]
- Götze S., Matoo O.B., Beniash E., Saborowski R., Sokolova I.M. Interactive effects of CO2 and trace metals on the proteasome activity and cellular stress response of marine bivalves Crassostrea virginica and Mercenaria mercenaria. Aquat. Toxicol. 2014;149:65–82. doi: 10.1016/j.aquatox.2014.01.027. [DOI] [PubMed] [Google Scholar]
- Gouin T., Armitage J.M., Cousins I.T., Muir D.C., Ng C.A., Reid L., Tao S. Influence of global climate change on chemical fate and bioaccumulation: the role of multimedia models. Environ. Toxicol. Chem. 2013;32(1):20–31. doi: 10.1002/etc.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnoll R. Factors affecting the distribution and behaviour of the crab Dotilla fenestrata on East African shores. Estuar. Coast Mar. Sci. 1973;1(2):137–152. [Google Scholar]
- Hoegh-Guldberg O., Cai R., Poloczanska E.S., Brewer P.G., Sundby S., Hilmi K., Fabry V.J., Jung S., Skirving W., Stone D.A. 2014. The Ocean. [Google Scholar]
- Hooper M.J., Ankley G.T., Cristol D.A., Maryoung L.A., Noyes P.D., Pinkerton K.E. Interactions between chemical and climate stressors: a role for mechanistic toxicology in assessing climate change risks. Environ. Toxicol. Chem. 2013;32(1):32–48. doi: 10.1002/etc.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz R., Borell E.M., Fine M., Shaked Y. Trace element profiles of the sea anemone Anemonia viridis living nearby a natural CO2 vent. PeerJ. 2014;2:e538. doi: 10.7717/peerj.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanina A.V., Beniash E., Etzkorn M., Meyers T.B., Ringwood A.H., Sokolova I.M. Short-term acute hypercapnia affects cellular responses to trace metals in the hard clams Mercenaria mercenaria. Aquat. Toxicol. 2013;140:123–133. doi: 10.1016/j.aquatox.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Ivanina A.V., Sokolova I.M. Interactive effects of metal pollution and ocean acidification on physiology of marine organisms. Curr. Zool. 2015;61(4):653–668. [Google Scholar]
- Kleypas J.A., Feely R.A., Fabry V.J., Langdon C., Sabine C.L., Robbins L.L. 2005. Impacts of ocean acidification on coral reefs and other marine calcifiers: a guide for future research. Report of a Workshop Held. [Google Scholar]
- Kumar M., Ferguson G., Xiao Y., Hooper G., Venema S. Studies on reproductive biology and distribution of blue swimmer crab (Portunus pelagicus) in South Australian Waters. Sardi Res. Rep. Ser. 2000;47:1324–2083. [Google Scholar]
- Lewis C., Ellis R.P., Vernon E., Elliot K., Newbatt S., Wilson R.W. Ocean acidification increases copper toxicity differentially in two key marine invertebrates with distinct acid-base responses. Sci. Rep. 2016;6:21554. doi: 10.1038/srep21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López I.R., Kalman J., Vale C., Blasco J. Influence of sediment acidification on the bioaccumulation of metals in Ruditapes philippinarum. Environ. Sci. Pollut. Control Ser. 2010;17(9):1519–1528. doi: 10.1007/s11356-010-0338-7. [DOI] [PubMed] [Google Scholar]
- Maitland D.P. Crabs that breathe air with their legs-Scopimera and Dotilla. Nature. 1986;319(6053):493. [Google Scholar]
- Millero F.J., Pierrot D., Lee K., Wanninkhof R., Feely R., Sabine C.L., Key R.M., Takahashi T. Dissociation constants for carbonic acid determined from field measurements. Deep Sea Res. Oceanogr. Res. Pap. 2002;49(10):1705–1723. [Google Scholar]
- Millero F.J., Woosley R., Ditrolio B., Waters J. Effect of ocean acidification on the speciation of metals in seawater. Oceanography. 2009;22(4):72–85. [Google Scholar]
- Noyes P.D., McElwee M.K., Miller H.D., Clark B.W., Van Tiem L.A., Walcott K.C., Erwin K.N., Levin E.D. The toxicology of climate change: environmental contaminants in a warming world. Environ. Int. 2009;35(6):971–986. doi: 10.1016/j.envint.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Núñez-Nogueira G., Fernández-Bringas L., Ordiano-Flores A., Gómez-Ponce A., de León-Hill C.P., González-Farías F. Accumulation and regulation effects from the metal mixture of Zn, Pb, and Cd in the tropical shrimp Penaeus vannamei. Biol. Trace Elem. Res. 2012;150(1-3):208–213. doi: 10.1007/s12011-012-9500-z. [DOI] [PubMed] [Google Scholar]
- Paez-Osuna F., Perez-Gonzalez R., Izaguirre-Fierro G., Zazueta-Padilla H., Flores-Campana L. Trace metal concentrations and their distribution in the lobster Panulirus inflatus (Bouvier, 1895) from the Mexican pacific coast. Environ. Pollut. 1995;90(2):163–170. doi: 10.1016/0269-7491(94)00103-k. [DOI] [PubMed] [Google Scholar]
- Paquin P.R., Santore R.C., Wu K.B., Kavvadas C.D., Di Toro D.M. The biotic ligand model: a model of the acute toxicity of metals to aquatic life. Environ. Sci. Pol. 2000;3:175–182. [Google Scholar]
- Pascal P.-Y., Fleeger J.W., Galvez F., Carman K.R. The toxicological interaction between ocean acidity and metals in coastal meiobenthic copepods. Mar. Pollut. Bull. 2010;60(12):2201–2208. doi: 10.1016/j.marpolbul.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Rainbow P.S. Trace metal bioaccumulation: models, metabolic availability and toxicity. Environ. Int. 2007;33(4):576–582. doi: 10.1016/j.envint.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Raven J., Caldeira K., Elderfield H., Hoegh-Guldberg O., Liss P., Riebesell U., Shepherd J., Turley C., Watson A. The Royal Society; 2005. Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide. [Google Scholar]
- Reisch D.L., Oshida P.S. FAO Fisheries Technical Paper. FAO; 1987. Manual of methods in aquatic environment research; part 10-short-term static biossays. [Google Scholar]
- Riba I., Garcia-Luque E., Blasco J., DelValls T. Bioavailability of heavy metals bound to estuarine sediments as a function of pH and salinity values. Chem. Speciat. Bioavailab. 2003;15(4):101–114. [Google Scholar]
- Rius M., Branch G.M., Griffiths C.L., Turon X. Larval settlement behaviour in six gregarious ascidians in relation to adult distribution. Mar. Ecol. Prog. Ser. 2010;418:151–163. [Google Scholar]
- Roberts D.A., Birchenough S.N., Lewis C., Sanders M.B., Bolam T., Sheahan D. Ocean acidification increases the toxicity of contaminated sediments. Global Change Biol. 2013;19(2):340–351. doi: 10.1111/gcb.12048. [DOI] [PubMed] [Google Scholar]
- Sudharsan S., Seedevi P., Ramasamy P., Subhapradha N., Vairamani S., Shanmugam A. Heavy metal accumulation in seaweeds and sea grasses along southeast coast of India. J. Chem. Pharmaceut. Res. 2012;4(9):4240–4244. [Google Scholar]
- Vijayram K., Geraldine P. Regulation of essential heavy metals (Cu, Cr, and Zn) by the freshwater prawn Macrobrachium malcolmsonii (Milne Edwards) Bull. Environ. Contam. Toxicol. 1996;56(2):335–342. doi: 10.1007/s001289900049. [DOI] [PubMed] [Google Scholar]
- Vivier L. 2010. Macrobenthic Community and Ecotoxicological Status of the Nhlabane Estuary. [Google Scholar]
- Ward G., Parrish P. Vol. 185. Food & Agriculture Org; 1982. (Manual of Methods in Aquatic Environment Research). [Google Scholar]
- Wu J.-P., Chen H.-C. Metallothionein induction and heavy metal accumulation in white shrimp Litopenaeus vannamei exposed to cadmium and zinc. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005;140(3-4):383–394. doi: 10.1016/j.cca.2005.03.006. [DOI] [PubMed] [Google Scholar]