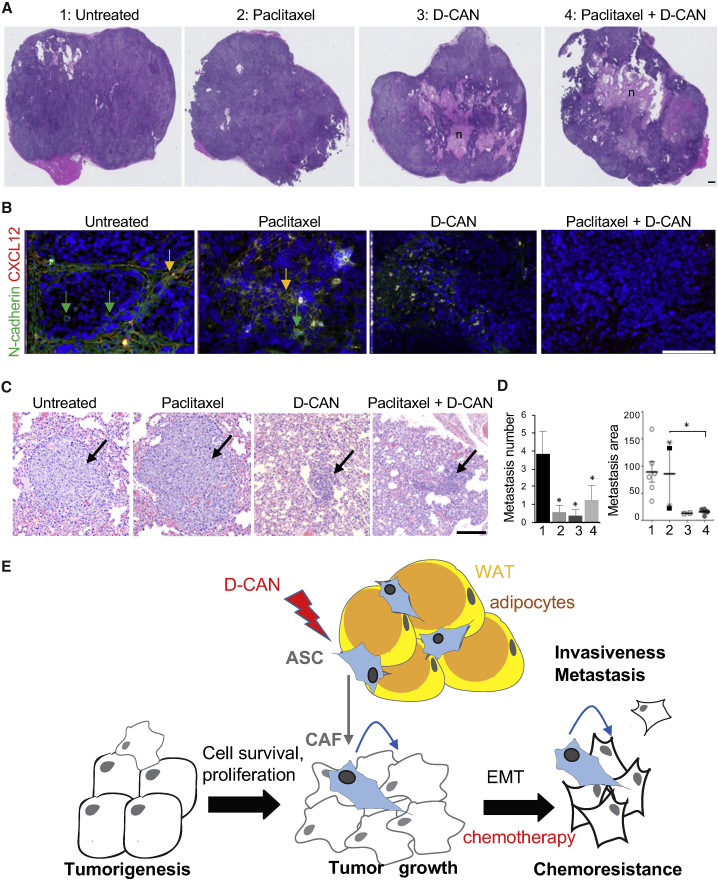
Figure 4.
ASC Depletion Suppresses Metastases in a Breast Cancer Xenograft Model
(A) H&E stainings of orthotopic SUM149 tumors grown in NSG mice injected with (1) PBS; (2) paclitaxel (7.5 mg/kg, intravenously [i.v.], 1/week); (3) D-CAN (1.1 mg/kg, subcutaneously [s.c.], 2/week); or (4) D-CAN + paclitaxel combination (N = 10/group). n, necrosis. (B) IF on tumor sections from mice in (A) showing reduction in CXCL12+ stromal cells and of N-cadherin+ cancer cells in tumors of mice treated with D-CAN. Nuclei are blue. (C) Micrographs of H&E-stained lung areas containing the largest metastases observed in mice from (A). (D) Quantification of metastases in mice from (C). Number: per lung; area: ImageJ pixels. Shown are mean ± SEM. ∗p < 0.05 (unpaired Student’s t test). Scale bars, 250 μm. (E) The model of the ASC role in cancer progression. ASCs derived from WAT and becoming CAFs initially increase the survival and proliferation of cancer cells and subsequently their EMT, chemotherapy resistance, invasiveness, and metastatic dissemination. Lightning bolt, D-CAN as an experimental therapeutic that can be used to deplete ASCs and suppress cancer progression.