Fig. 1.
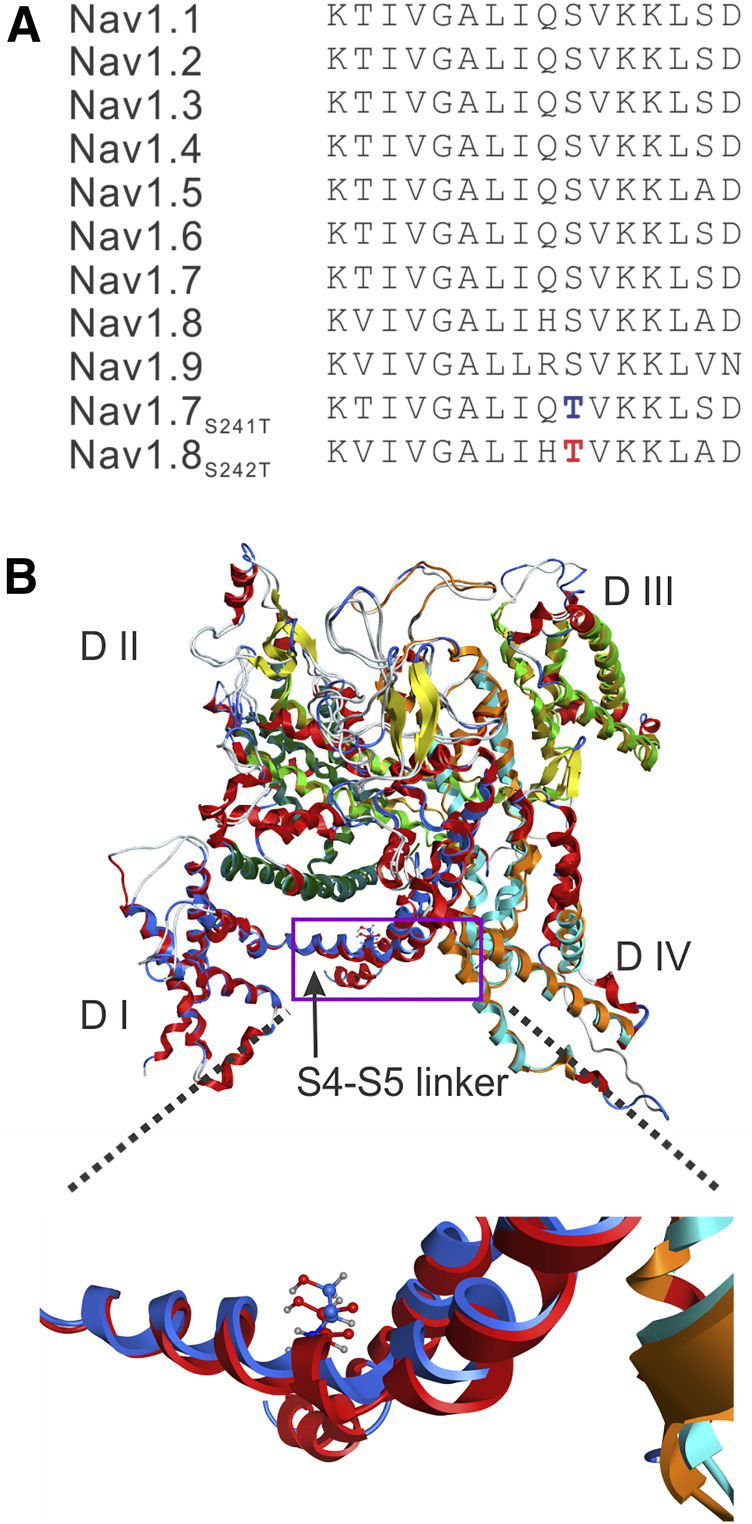
(A) Sequence alignment and position of mutation in the DI S4-S5 linker in the NaV1.8 channel. The sequence of the DI S4-S5 linker is invariant in the nine sodium channels from humans. The substitution of S242T is highlighted in red type, and the substitution of NaV1.7-S241T is highlighted in blue type. (B) Structural modeling of human NaV1.8 and human NaV1.7 channels. The two channel structures were superimposed, and the membrane-spanning segments showed good alignment. DI is colored blue in NaV1.8, and red in NaV1.7. The overall structure is tilted and rotated to give a clearer view of the DI S4-S5 linker where the S242T and NaV1.7-S241T substitution occurs. A box is drawn to indicate the region of the structure that is zoomed out to better show the NaV1.8-T242 residue (blue carbons) and NaV1.7-T241 residue (red carbons). The serine-to-threonine substitution in the DI S4-S5 linker in NaV1.7 and NaV1.8 maintains the same position and orientation of the side chain.