Fig 1.
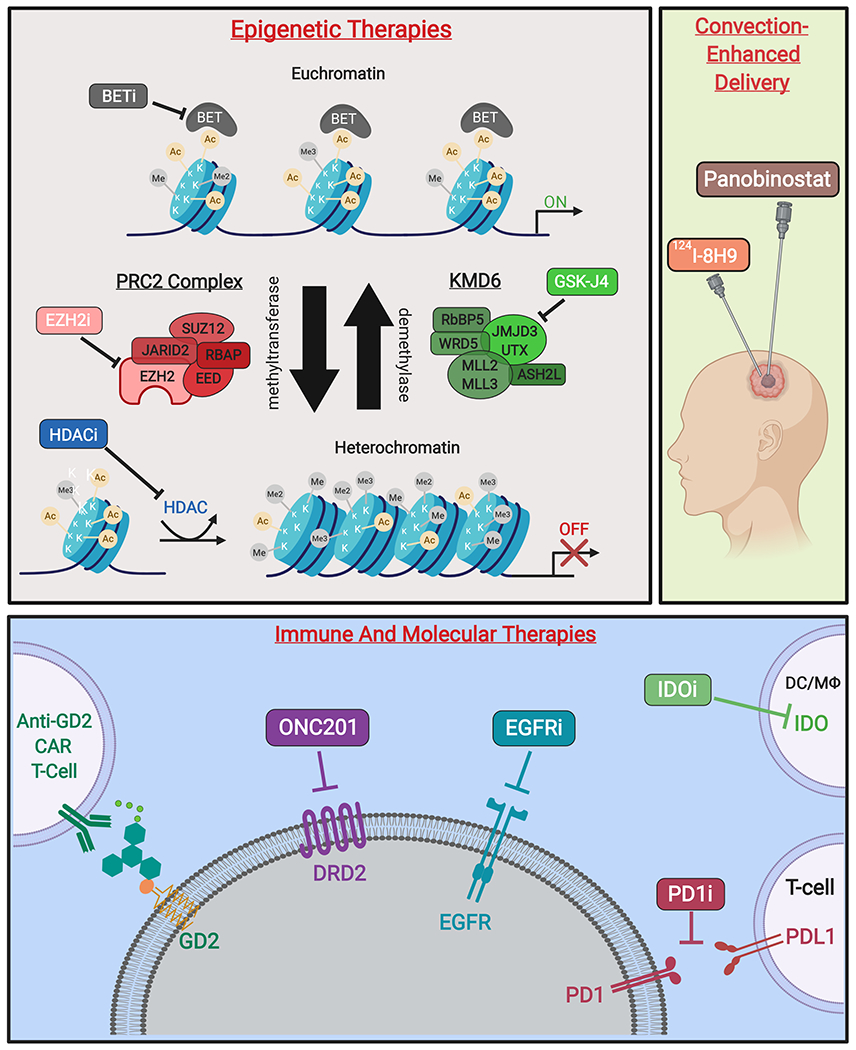
Novel therapies targeting H3K27M-mutant gliomas. (A)* Epigenetic therapies modify transcriptional activity at the level of chromatin. Competitive inhibition of BET family proteins reduce recognition of acetylated histones and subsequent transcription initiation. Additionally, inhibitors of EZH2 activity prevent formation of heterochromatin by inactivating the catalytic subunit of the PRC2 methyltransferase complex. Moreover, non-specific HDAC inhibitors reduce heterochromatin formation. Finally, prodrug GSK-J4/J1 increases H3K27 methylation by inhibition of K27-demethylases JMJD3 and UTX. (B) Therapeutic agents delivered by CED include omburtamab, a radioactive iodine isotope conjugated to the anti-glioma monoclonal antibody 8H9 (124I-8H9), which has undergone phase I evaluation [58]. Additionally, delivery of panobinostat nanoparticle formulation MTX110 via CED is ongoing (Phase I/II, PNOC015). (C) Emerging immune therapies include CAR T-cells against GD2, a disialoganglioside highly expressed in H3K27M gliomas. PD1/PDL1 immune checkpoint inhibitors prevent T-cell suppression, while IDO inhibitors increase T-cell activation at the site of tumors cells. Molecular therapies include small-molecule DRD2-antagonist ONC201, as well as inhibitors of EGFR to reduce tumor growth and proliferation [59].
Figure created with BioRender.com. *Figure 1A adapted from Hashizume (2017) [25].
