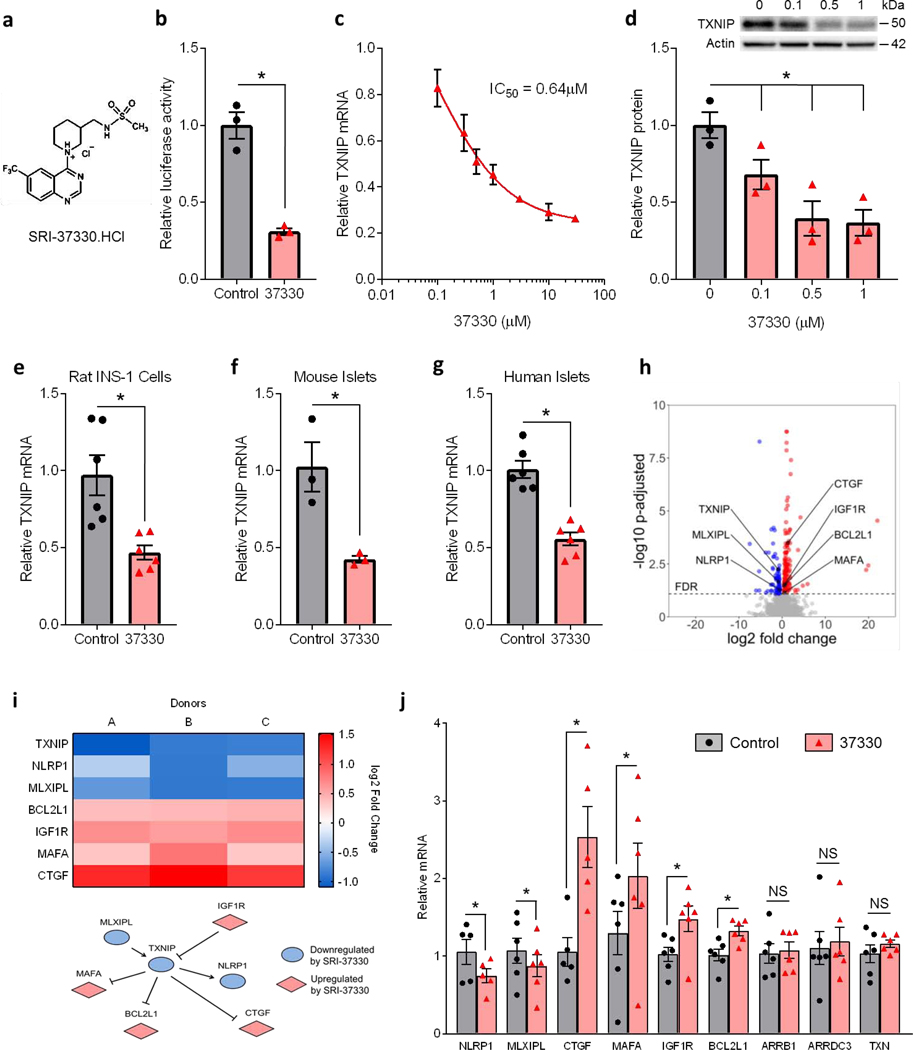
Figure 1. Substituted quinazoline sulfonamide (SRI-37330) controls islet TXNIP expression and associated signaling pathway genes.
(a) Chemical structure of the substituted quinazoline sulfonamide, SRI-37330 (the HCl salt is shown).
(b) Effect of SRI-37330 on the activity of the full-length human TXNIP promoter as assessed by dual luciferase and compared to no SRI-37330 control (t2 = 6.71, *P = 0.0215).
(c) Inhibition of TXNIP mRNA expression in INS-1 cells by SRI-37330 as assessed by qPCR 7-point dose response curve.
(d) Dose-dependent inhibition of TXNIP protein expression in INS-1 cells by SRI-37330 as assessed by immunoblotting (F3,8 = 9.65, *P = 0.0049); a representative immunoblot (n = 3 total blots analyzed) is shown.
(e) SRI-37330 effects on TXNIP expression in the context of high (25 mM) glucose in rat INS-1 cells (t10 = 3.63, *P = 0.0046) as assessed by qPCR; mean ± s.e.m., n = 6 independent experiments.
(f) SRI-37330 effects on TXNIP expression in the context of high glucose in primary mouse islets (t4 = 3.67, *P = 0.0213), n = 3 independent experiments.
(g) SRI-37330 effects on TXNIP expression in the context of high glucose in human islets (t5 = 9.11, *P = 0.0003), n = 6 different human islet donors.
(h) RNA sequencing was performed on human islets from three different donors (A-C) treated with or without SRI-37330 in the context of high (25 mM) glucose. Volcano plot contains all genes with a baseMean expression of > 5. Genes with a FDR <0.05 are shown in color.
(i) Heat map and pathway showing TXNIP-associated genes that are significantly (adjusted DESeq2 P< 0.05) changed after treatment with SRI-37330.
(j) Confirmation by qPCR of changes in the expression of TXNIP-associated genes (NLRP1 *P = 0.0374, MLXIPL *P = 0.0309, CTGF *P = 0.0031, MAFA *P = 0.0075, IGF1R *P = 0.0061, BCL2L1 *P = 0.0004). The arrestin proteins ARRB1 and AADC3 as well as thioredoxin were run as negative controls (N.S. not significant), n = 6 human islet donors.