Abstract
Low- and middle-income countries (LMICs) remain neglected in the Coronavirus 19 (COVID-19) pandemic. COVID-19 convalescent plasma (CCP) (i.e. plasma collected from individuals after their recovery from COVID-19) has emerged as a leading medical treatment for COVID-19. Studies to date support the safety—and increasingly the efficacy—of CCP to treat COVID-19. This has motivated large-scale procurement and transfusion of CCP, notably in the United States (US), where inventories of CCP have been attained, and government-supported stockpiling of CCP is underway. CCP is a therapy that could be implemented in LMICs. However, systemic and transfusion-specific challenges (e.g. capacity for donor mobilization and collections) impede local procurement of this resource in sufficient volumes to meet clinical demand. This raises the question as to whether there are strategies to facilitate sharing of CCP with LMICs and/or bolstering local capacity for collection to contend with the health crisis. While compelling, there are cost-related, logistical and regulatory barriers to both approaches. For one, there is complexity in diverting national interest (e.g. in the US) away from an epidemic that displays few signs of abating. There are also concerns regarding equitable distribution of CCP in LMICs and how that might be overcome. Further, the barriers to blood donation in general apply to collection of CCP; these obstacles are longstanding, accounting for the inability of many LMICs to meet their blood transfusion needs. Nonetheless, CCP affords dual opportunity for humanitarian outreach while tackling a broader challenge of blood transfusion safety and availability.
Keywords: COVID-19, SARS-CoV-2, COVID-19 serotherapy, Blood transfusion, Blood donors, Global health
1. Overview of convalescent plasma to treat COVID-19
Coronavirus Disease 2019 (COVID-19) convalescent plasma (CCP) (i.e. plasma collected from individuals after their recovery from COVID-19), has emerged as a leading treatment for COVID-19. This is most notable in the United States (US) where a national, expanded access program was devised to scale up collections and transfusions of CCP for hospitalized patients with severe or life threatening COVID-19 [1]. More modest collection of CCP outside of the US has been undertaken —primarily— to support clinical trials [2]. CCP has been found to be safe conferring comparable risk to non-immune plasma transfusion [3]. Further, observational studies [[3], [4], [5], [6], [7], [8], [9], [10], [11]] and increasingly clinical trials suggest that it is an effective treatment of COVID-19, particularly when administered early in the disease process [12,13].
2. Access of COVID-19 convalescent plasma in low- and middle- income countries
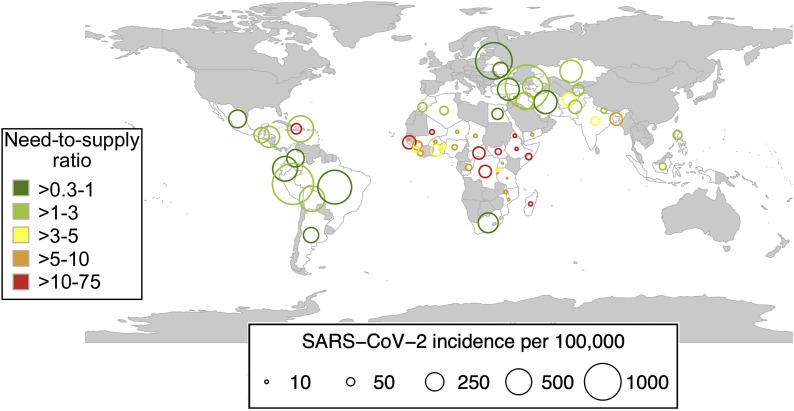
The outlook for many low- and middle- income countries (LMICs) is somber. Globally, eight of the 10 countries with the highest numbers of new cases of COVID-19 are either upper middle income- (i.e. Peru, Russia, South Africa, Argentina, Colombia, Mexico, Brazil) or lower-middle income countries (i.e. India) [14,15]. Low-income countries are conspicuously absent from the list of most affected. Rather than offering an encouraging sign, it is just the opposite, highlighting the limited capacity for testing and case reporting in low-income countries [16]. Indeed, only 1 % of all reported cases worldwide originate in low-income countries [15]. Broadly, the COVID-19 pandemic is underway in countries that lack the capacity to contend with the health crisis. Beyond the systemic challenges of resource poor settings, the procurement of CCP requires an optimally functioning blood collection system. Many —if not most—LMICs that report high numbers of reported cases of COVID-19, lack sufficient blood supply to contend with the clinical need for transfusion (Fig. 1 ) [17]. The challenges pertaining to provision of a safe and adequate blood supply in LMICs pre-date COVID-19, and span donor mobilization, collections, processing, infectious testing and transfusion itself [[17], [18], [19]]. These general obstacles apply, similarly to the procurement of CCP [2]. Limited capacity for testing impedes the ability to identify suitable donors for CCP. Plasmapheresis, the major mode of CCP collection in HICs, is limited in LMICs given the associated high equipment costs and requirement for technical expertise of operation [20]. Plasmapheresis is a highly efficient mode of collection, whereby up to 800 mL (the equivalent of 3 or 4 units) of CCP can be produced from a single collection [21]. Individuals can also donate frequently (e.g. weekly) given the minimal red blood cell loss. By contrast, whole blood collection, the mainstay of collection in LMICs, requires longer deferral periods (∼8 weeks).
Fig. 1.
Bubble plot displaying the numbers of reported COVID-19 cases as expressed per 100,000 population in the low and middle-income countries (top 30 each) that report the highest numbers of COVID -19 cases (July 7, 2020). The size of the bubble corresponds to the number of cases per 100,000. The color of the bubble corresponds to the baseline blood need to supply ratio in these countries reporting the highest numbers of COVID-19 cases. Green reflects a more favorable need:supply ratio (<1) while red depicts an unfavorable supply (>10). Countries that are not plotted are in greyscale. (baseline blood need to supply ratio is adapted from Roberts N, James S, et al Lancet Haematol 2019;6:e606-e15 [17]).
Given limited capacity for procurement of CCP in LMICs and an extant inventory and capability to increase collection CCP in high-income countries (HICs), this raises the question as to whether there is scope for redistribution of CCP to countries in need.
3. Barriers to access to CCP
While the notion of a humanitarian response to share CCP with LMICs is compelling, there are formidable barriers to consider. Foremost, CCP remains an investigational blood product, whereby most countries still restrict CCP use to clinical trials, reserving judgments of efficacy until clearly shown by clinical trial. Second, is the regulatory complexity of transporting an investigational human blood product across international borders. National biological product regulations vary widely, requiring careful study to ensure that the exported product meets the receiving country’s regulatory requirements, and a reliable system exists for timely and traceable distribution of this resource. The long delays that are often reported for medications making their way through foreign customs offices could render CCP inadmissible. Countries that participate in international stem cell registries may be better poised to meet such a challenge, given existing mechanisms that permit shipping of stem cells across borders [22]. The investigational nature of CCP may also present a regulatory hurdle.
Third, the cost of collection and shipping of CCP is prohibitive to most LMICs. The reimbursement for a single unit of CCP in the US is over $US700; this far exceeds the cost of a local acquisition in a LMIC. There are also stringent regulations to ship biological agents such as CCP, and international air freight of plasma is expensive requiring packing of the units on dry ice [23]. Assuming bulk shipment, the per unit shipping cost of CCP would still be approximately $US100, tax and/or custom duties, notwithstanding. Given that many blood transfusion systems in LMICs rely on external funding, it is unlikely that they will be able to pay [20]. Blood centers in the US have long been under financial strain and can’t sustain an uncompensated loss [24]. In the absence of a defined sponsor or philanthropic effort, the feasibility becomes questionable.
Fourth, the trajectory of the pandemic is uncertain. Rather than flattening of the epidemic curve as was suggested in late April 2020, record numbers of cases were reported in the US through June and July 2020, particularly in parts of the country that were previously less affected (e.g. California, Florida and Arizona) [15]. Similarly, a resurgence has been reported in other countries where the virus had seemed to have been under control. This will motivate for retention of extant inventories in anticipation of the continued surge in new cases as well as seasonal outbreaks and/or long-term persistence of SARS-CoV-2. Local CCP reserves may be needed chronically until durable, population-based preventive strategies (e.g. vaccines) become available. There has also been significant investment in developing the systems to ensure adequate supply of CCP; this could obligate local collectors to those sponsors. In short, prioritization of national interests and the reluctance to share inventory does not bode well for LMICs in need.
Fifth, available inventories of CCP are insufficient to address the needs of all countries. This introduces an ethical challenge of equitable distribution. Specifically, it raises the questions as to how one decides on which countries to support and the quantities for sharing. Even within a given country, how would the units be deployed? The approach to selection must be carefully considered, balancing the need (i.e. as reflected by the epidemiology of COVID-19 in the recipient country), local capacity for donor testing and procurement of CCP, scope for treatment in the absence of CCP (e.g. availability of mechanical ventilation) as well as the political and regulatory environment. There is regional variability in all of these elements.
4. Potential solutions
The attention on CCP affords a dual opportunity: to tackle COVID-19 while addressing a long-neglected problem of a global transfusion deficit. A massive collaborative effort spanning government, prominent academic institutions, the blood collection agencies and professional bodies, philanthropic organizations and commercial entities, (e.g. in the technology sector) has assured a pipeline of CCP in the US and other HICs [1]. National interest could be directed more broadly, through limited sharing of CCP and/or building local capacity in LMICs. While daunting, it is important to avoid being entangled in the web of impossibility.
To address questions of efficacy, pairing trials in high-income countries (HICs) with those of similar design to those in LMICs with a high incidence of COVID-19, would expedite subject accrual and attainment of enrollment goals [25]. At least two trials have already failed to meet enrollment targets due to waning local epidemics [12,13]. Regulatory and transit barriers are soluble: bone marrow and hematopoietic stem cells are routinely couriered internationally; similarly international rare donor programs have been used to exchange blood products and reagents, often in times of acute need [26,27].
An uncertain trajectory requires a more durable solution i.e. mobilization of internal resources to bolster local supplies of CCP. There are already published guidelines on procurement of CCP in LMICs that draw on ways to navigate obstacles such as use of whole blood collection for preparation of CCP in the absence of apheresis equipment [2,28]. There are examples where local capacity has been developed, rapidly, even in austere environments. During the 2014–2015 Ebola outbreak in Liberia, convalescent plasma was collected successfully, despite enormous challenges [29]. This highlights the ability to overcome barriers with creativity and drive. To this end, CCP affords opportunity. The President’s Emergency Plan for AIDS Relief (PEPFAR) program provided instrumental support to combat the AIDS crisis, yet also conferred a host of collateral benefits, bolstering health systems and advancing blood transfusion safety [30]. Other international governmental- (e.g. WHO), professional- (e.g. International Society of Blood Transfusion [ISBT], AABB) and philanthropic (e.g. Gates Foundation) organizations also have a critical role to play in facilitating these activities. Attention on CCP should be used to advance similar strategies to address the broader challenge of global blood transfusion safety and availability, drawing on the innovative approaches that have been employed successfully during this humanitarian crisis.
Funding
EMB’s effort is supported in part by the National Heart Lung and Blood Institute (1K23HL151826-01).
Declaration of Competing Interest
EMB reports personal fees and non-financial support from Terumo BCT, personal fees and non-financial support from Grifols Diagnostics Solutions, outside of the submitted work; EMB is a member of the United States Food and Drug Administration (FDA) Blood Products Advisory Committee. Any views or opinions that are expressed in this manuscript are that of the author's, based on his own scientific expertise and professional judgment; they do not necessarily represent the views of either the Blood Products Advisory Committee or the formal position of FDA, and also do not bind or otherwise obligate or commit either Advisory Committee or the Agency to the views expressed.
References
- 1.COVID-19 expanded access program 2020.
- 2.Bloch E.M., Goel R., Wendel S., Burnouf T., Al-Riyami A.Z., Ang A.L., et al. Guidance for the procurement of COVID-19 convalescent plasma: differences between high- and low-middle-income countries. Vox Sang. 2020 doi: 10.1111/vox.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joyner M., Bruno K., Stephen A., Klassen S., Kunze K., Johnson P., et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020 doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salazar E., Perez K.K., Ashraf M., Chen J., Castillo B., Christensen P.A., et al. Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol. 2020 doi: 10.1016/j.ajpath.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hegerova L., Gooley T., Sweerus K.A., Maree C.L., Bailey N., Bailey M., et al. Use of convalescent plasma in hospitalized patients with Covid-19 - case series. Blood. 2020 doi: 10.1182/blood.2020006964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia X., Li K., Wu L., Wang Z., Zhu M., Huang B., et al. Improved clinical symptoms and mortality on severe/critical COVID-19 patients utilizing convalescent plasma transfusion. Blood. 2020 doi: 10.1182/blood.2020007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abolghasemi H., Eshghi P., Cheraghali A.M., Imani Fooladi A.A., Bolouki Moghaddam F., Imanizadeh S., et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apher Sci. 2020 doi: 10.1016/j.transci.2020.102875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyner M.J., Klassen S.A., Senefeld J., Johnson P.W., Carter R.E., Wiggins C.C., et al. Evidence favouring the efficacy of convalescent plasma for COVID-19 therapy. medRxiv. 2020 2020.07.29.20162917. [Google Scholar]
- 11.Joyner M.J., Senefeld J.W., Klassen S.A., Mills J.R., Johnson P.W., Theel E.S., et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv. 2020 2020.08.12.20169359. [Google Scholar]
- 12.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020 doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avendano-Sola C., Ramos-Martinez A., Munez-Rubio E., Ruiz-Antoran B., Malo de Molina R., Torres F., et al. Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. medRxiv. 2020 2020.08.26.20182444. [Google Scholar]
- 14.2020. World Bank country and lending groups. The World Bank group. [Google Scholar]
- 15.JHU . The Center for Systems Science and Engineering (CSSE) at JHU; 2020. Coronavirus COVID-19 global cases by the center for systems science and engineering at johns hopkins. [Google Scholar]
- 16.Peplow M. Developing countries face diagnostic challenges as the COVID-19 pandemic surges. Chem Eng News. 2020 [Google Scholar]
- 17.Roberts N., James S., Delaney M., Fitzmaurice C. The global need and availability of blood products: a modelling study. Lancet Haematol. 2019;6 doi: 10.1016/S2352-3026(19)30200-5. e606-e15. [DOI] [PubMed] [Google Scholar]
- 18.Bloch E.M., Gehrie E.A., Ness P.M., Sugarman J., Tobian A. Blood transfusion safety in low-resourced countries: aspiring to a higher standard. Ann Intern Med. 2020 doi: 10.7326/M20-0203. [DOI] [PubMed] [Google Scholar]
- 19.Weimer A., Tagny C.T., Tapko J.B., Gouws C., Tobian A.A.R., Ness P.M., et al. Blood transfusion safety in sub-Saharan Africa: a literature review of changes and challenges in the 21st century. Transfusion. 2019;59:412–427. doi: 10.1111/trf.14949. [DOI] [PubMed] [Google Scholar]
- 20.WHO . 2017. Global status report on blood safety and availability 2016. Geneva, Switzerland. [Google Scholar]
- 21.Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L., et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AABB . 2020. International competent authorities. [Google Scholar]
- 23.WHO . 2020. Guidelines for the safe transport of infectious substances and diagnostic specimens. [Google Scholar]
- 24.Klein H.G., Hrouda J.C., Epstein J.S. Crisis in the sustainability of the U.S. Blood system. N Engl J Med. 2017;377:1485–1488. doi: 10.1056/NEJMsb1706496. [DOI] [PubMed] [Google Scholar]
- 25.Petkova E., Antman E.M., Troxel A.B. Pooling data from individual clinical trials in the COVID-19 era. JAMA. 2020 doi: 10.1001/jama.2020.13042. [DOI] [PubMed] [Google Scholar]
- 26.2020. SCARF: serum, cells and rare fluids exchange. [Google Scholar]
- 27.2020. Be the match: international donor centers. [Google Scholar]
- 28.Smid W., Burnouf T., Epstein J., Kamel H., Sibinga C., Somuah D., et al. Organizing Committee of the ISBT Working Party on Global Blood Safety; 2020. Points to consider in the preparation and transfusion of COVID-19 convalescent plasma in low- and middle- income countries. [Google Scholar]
- 29.Brown J.F., Rowe K., Zacharias P., van Hasselt J., Dye J.M., Wohl D.A., et al. Apheresis for collection of Ebola convalescent plasma in Liberia. J Clin Apher. 2017;32:175–181. doi: 10.1002/jca.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ifland L., Bloch E.M., Pitman J.P. Funding blood safety in the 21st century. Transfusion. 2018;58:105–112. doi: 10.1111/trf.14374. [DOI] [PubMed] [Google Scholar]