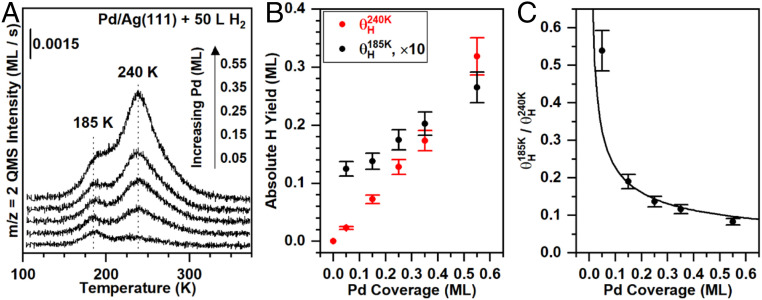
Fig. 3.
The palladium coverage affects the yields of hydrogen atoms which desorb in the two different peaks at a fixed dihydrogen exposure, providing additional evidence that the 240 K peak is due to recombination from palladium islands and the 185 K peak is associated with exposed silver. (A) Temperature-programmed desorption of dihydrogen as a function of palladium coverage on Ag(111) (θPd = 0.05 to 0.55 ML) for a fixed dihydrogen exposure of 50 L. For reference, at a palladium coverage of 0.45 ML and a dihydrogen exposure of 50 L, θH/Pd is ∼50% of the highest coverage achieved (Fig. 2B). (B) The absolute yields of dihydrogen in the 240 K peak (red) and the 185 K peak (black) increase nearly linearly, albeit with different slopes. The absolute yield of hydrogen atoms on silver is scaled by a factor of 10. (C) The ratio of coverages of the hydrogen atoms from the 185 K peak to those from the 240 K peak indicates that the 185 K peak is more readily populated for low palladium coverages. The solid curve illustrates the relationship between the palladium coverage and the palladium−silver interface length, assuming hexagonal palladium islands of uniform size (SI Appendix).